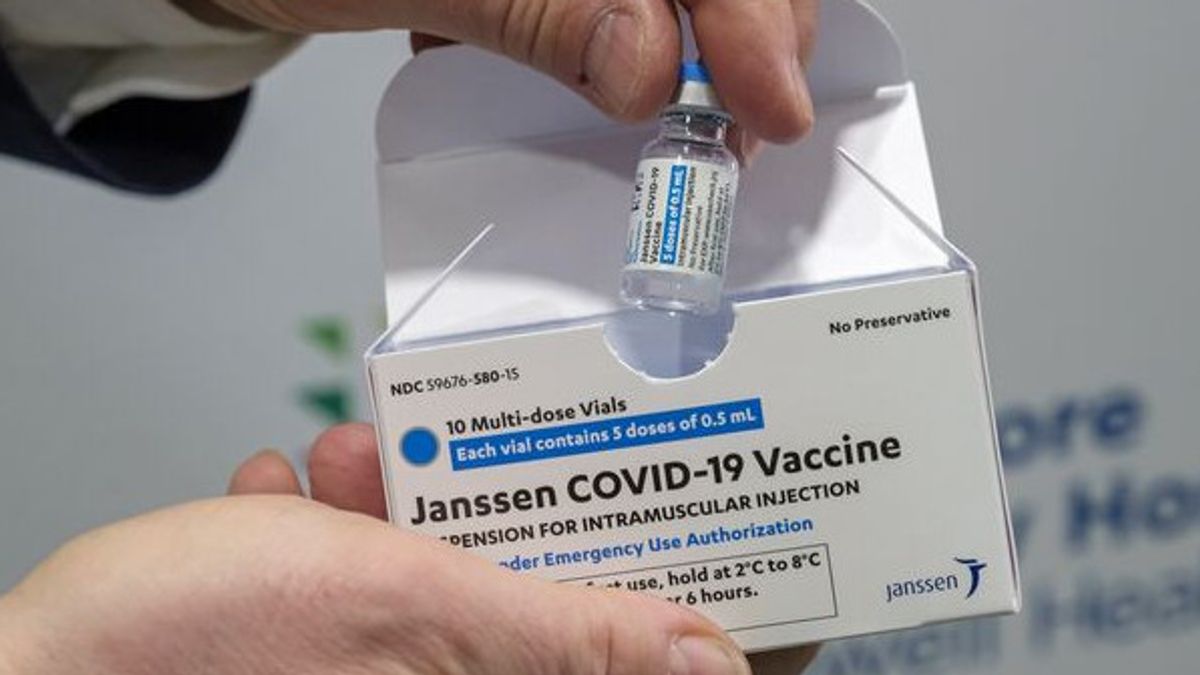
JAKARTA - The COVID-19 vaccine manufacturer Johnson & Johnson announced that they will continue the vaccine launch program in Europe. This came after European health regulators said the benefits of the vaccine outweighed the risk of a very rare blood clot.
The European health regulator, the European Medicines Agency (EMA) on Tuesday 20 April recommended adding a warning about rare blood clots with low blood platelet counts to the label of Johnson & Johnson vaccine products.
The Use of the Johnson & Johnson vaccine was temporarily suspended by US regulators last week after a rare cerebral blood clot combined with low blood platelet counts was reported in six women. That condition made the manufacturer postpone the launch in Europe.
Johnson & Johnson explained that the label will include warnings about the risk of rare side effects, as well as for instructions on how to recognize and treat them. Submissions will also be made for the European Union, Norway, and Iceland, as well as continuing clinical trial work. Meanwhile, the Netherlands is known to start using this vaccine on Wednesday, April 21.
"This is a very rare event. We hope that by making people aware and implementing clear diagnostic and therapeutic guidelines so that we can restore confidence in our vaccine", said Johnson & Johnson Chief Scientific Officer Paul Stoffels.
Separately, the United States Centers for Disease Control and Prevention (CDC) and the United States Food and Drug Administration (FDA) is also reviewing the rare blood clots reported in people who have had the injection. The advisory committee will meet on Friday and can make recommendations.
"The results from the vaccine review are important for the overall global vaccination effort, given the Johnson & Johnson vaccine does not have the extreme cold storage requirements of the mRNA vaccine", said analyst Edward Jones Ashtyn Evans, referring to vaccines from Moderna and Pfizer BioNTech.
SEE ALSO:
To note, the Johnson & Johnson vaccine can be stored at normal refrigerator temperatures and is expected to be used worldwide.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)