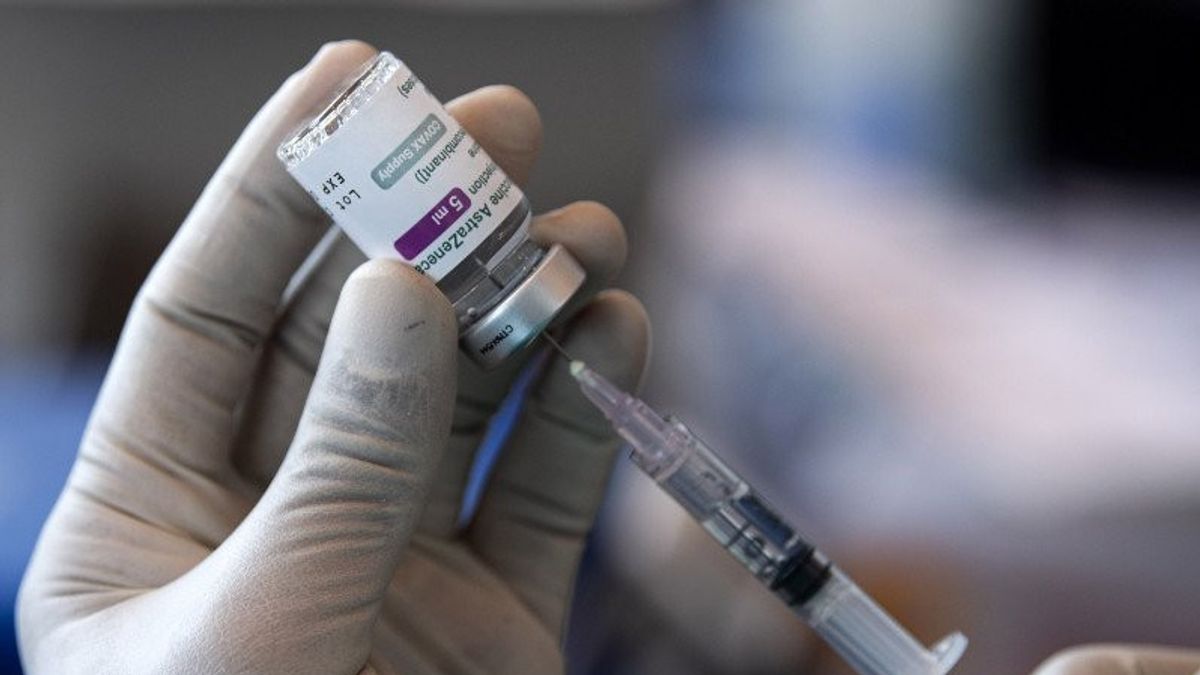
JAKARTA - The DKI Jakarta Provincial Government has decided to continue vaccination using the AstraZeneca brand with the target community in slum neighborhoods after a case of youth who died after being vaccinated with that vaccine.
However, one batch number CTMAV547 is not used. This batch is a vaccine group, one of which is injected into the young man. Currently, AstraZeneca batch CTMAV547 is being researched.
"The implementation of the 1st dose vaccination with AstraZeneca starting today can be continued using vaccines other than the batch number CTMAV547", Head of DKI Health Office Widyastuti said in her statement, Tuesday, May 18.
Widyastuti asked her staff to forward information to health centers and vaccine centers or health facilities that provide vaccination services.
Then, vaccination service officers are asked to ensure that vaccine participants receive clear information about the telephone number of officers who can be contacted if they experience complaints.
"Ensure that the officer's telephone number on the vaccine card can be contacted and give a good response", said Widyastuti.
If vaccination participants experience post-vaccine health complaints, they can use paracetamol drugs. The officer was asked to advise participants to check with the nearest health facility if the complaints got worse.
"Health facilities receiving vaccine participants with Post-immunization follow-up events (KIPI) complaints must provide a strong medical response and immediately forward the information to Public health center (puskesmas) in the region", she explained.
For information, a 22-year-old youth from Buaran, East Jakarta, named Trio Fauqi Virdaus died after being injected with the AstraZeneca vaccine batch CTMAV547 the day before.
SEE ALSO:
Initially, Trio felt symptoms of fever after receiving the AstraZeneca vaccine injection at Istora Senayan on Wednesday, May 5.
The next day, Trio's condition weakened. He was then taken to the hospital. However, Trio's life could not be saved. Trio was declared dead on Thursday, May 6 at around 12.30 p.m. local time.
Finally, the Ministry of Health temporarily stopped the use of a production batch or batch of AstraZeneca vaccine type CTMAV547.
Public Relations of the Ministry of Health, Widyawati, said that a batch of AstraZeneca vaccines that were suspended is currently being tested for toxicity and sterility by the Food and Drug Supervisory Agency (BPOM).
"Not all of the AstraZeneca vaccine batches have been stopped for distribution and use. Only the CTMAV547 batch is temporarily suspended pending the results of investigations and testing from BPOM which may take one to two weeks", said Widyawati in her statement, Sunday, May 16.
This batch of CTMAV547 totaled 448.480 doses and was part of 3.852.000 doses of AstraZeneca. This vaccine was received by Indonesia on April 26, 2021, through the Covax Facility scheme with WHO. This batch has been distributed to the National Army (TNI) and partly to DKI Jakarta and North Sulawesi.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)