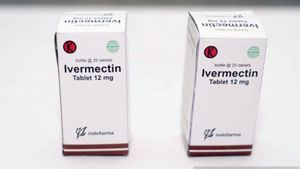
JAKARTA - Minister of State-Owned Enterprises (BUMN) Erick Thohir promised to provide cheap therapeutic drugs to prevent or cure COVID-19. This includes Ivermectin which has obtained Approval for Clinical Trials (PPUK) from the Food and Drug Supervisory Agency (BPOM).
Later, after clinical trials of the Ivermectin drug for COVID-19 patients are carried out, BUMN through its pharmaceutical holding company PT Indofarma Tbk will market it to the public at affordable prices. It is planned that this drug will be priced between IDR 5.000 to IDR 7.000 per tablet.
"With cheap and affordable drug prices, I'm sure people will be able to get them easily and won't be a burden. Especially for the prevention of COVID-19, you don't need to always take it and only take 2-3 tablets. Likewise for healing", said Erick in his written statement quoted on Tuesday, June 29.
He said the provision of COVID-19 therapeutic drugs at low prices was indeed a concern for the government. The hope is that the community will not be burdened with expensive drug prices and can prioritize buying basic needs in the midst of this pandemic.
SEE ALSO:
Furthermore, Erick said that the administration of PPUK from BPOM for Ivermectin as a COVID-19 therapeutic drug would be a change so that Indonesia could control this pandemic.
He ensured that his party was ready to mass-produce Ivermectin in a short time when clinical trials were completed. "In terms of infrastructure, we are ready to mass-produce Ivermectin. This drug will become a cheap therapeutic drug for the people, especially since Indofarma has prepared production of 4.5 million tablets per month", said Erick.
"If the BPOM clinical trial is completed and the distribution permit has been issued as a sign that the Ivermectin drug is good for all of us, then we will boost this production in order to quickly reduce the positive cases of COVID-19", he added.
As previously reported, the BPOM has given PPUK to Ivermectin, which was previously circulating for worming indications or worm medicine in certain doses.
This test was carried out by giving Ivermectin for five days to COVID-19 patients and then observing it for 28 days. The clinical trial will last for approximately three months with the provision of reports every month.
Meanwhile, the location of the Ivermectin clinical trial will be carried out in eight hospitals, namely Persahabatan Hospital Jakarta, Sulianti Saroso Hospital Jakarta, Dr. Soedarso Hospital Pontianak, Adam Malik General Hospital in Medan, and Gatot Subroto Army Hospital in Jakarta.
In addition, Dr. Esnawan Antariksa Hospital Jakarta, Dr. Suyoto Hospital Jakarta, and the COVID-19 Emergency Hospital Wisma Atlet Kemayoran Jakarta.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)