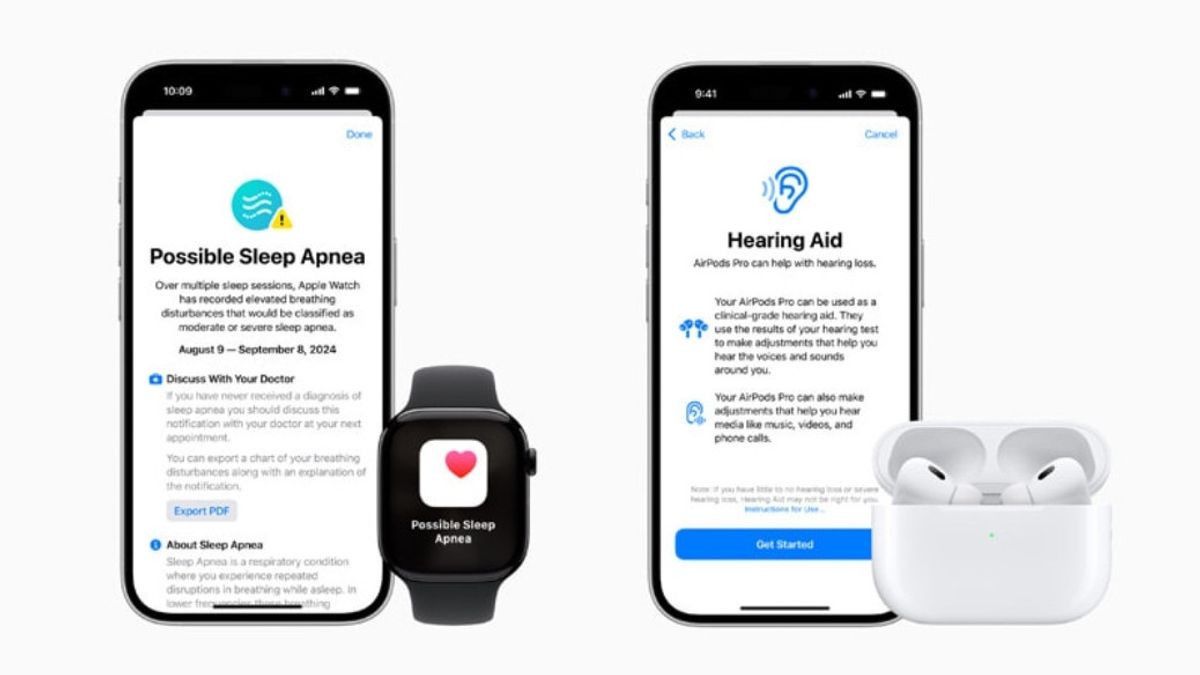
JAKARTA When launching the latest AirPods model, Apple revealed that they had added hearing aids to the AirPods Pro 2. When launched, this feature did not get permission.
Not even a week after its release, the US Food and Drug Administration (FDA) announced that it had allowed AirPods to sell with Hearing Aid Feature. Thanks to this permit, AirPods Pro 2 officially became the first software to have hearing aids.
Michelle Tarver, Director of the Center for Hearing and Health Radiology of the FDA, revealed that hearing loss is a health problem that continues to increase significantly. With this new innovation, people with hearing loss can be helped.
"The marketing authorization of hearing aids that are sold freely on consumer audio products that are widely used today is a step forward in the availability, accessibility, and acceptance of hearing aids for adults," said Tarver.
AirPods Pro 2 can be used by people with mild to moderate hearing loss. This new technology can be a safe, innovative, and effective solution because users can adjust their devices according to their needs.
SEE ALSO:
The FDA explains that Hearing Aid can be controlled without the help of hearing experts. Users can adjust their hearing levels through AirPods compatible devices, such as iPhones, iPads, and other connection devices.
Before being allowed to circulate on the market, the FDA has tested Hearing Aid to 118 subjects who have mild to moderate hearing problems. From the test results, it is proven that the technology Apple is developing provides tangible benefits.
"The FDA authorized the marketing authorization of Hearing Aid Feature to Apple Inc," the agency wrote on its official website. "No bad events (occurred) related to the devices observed in this study."
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)