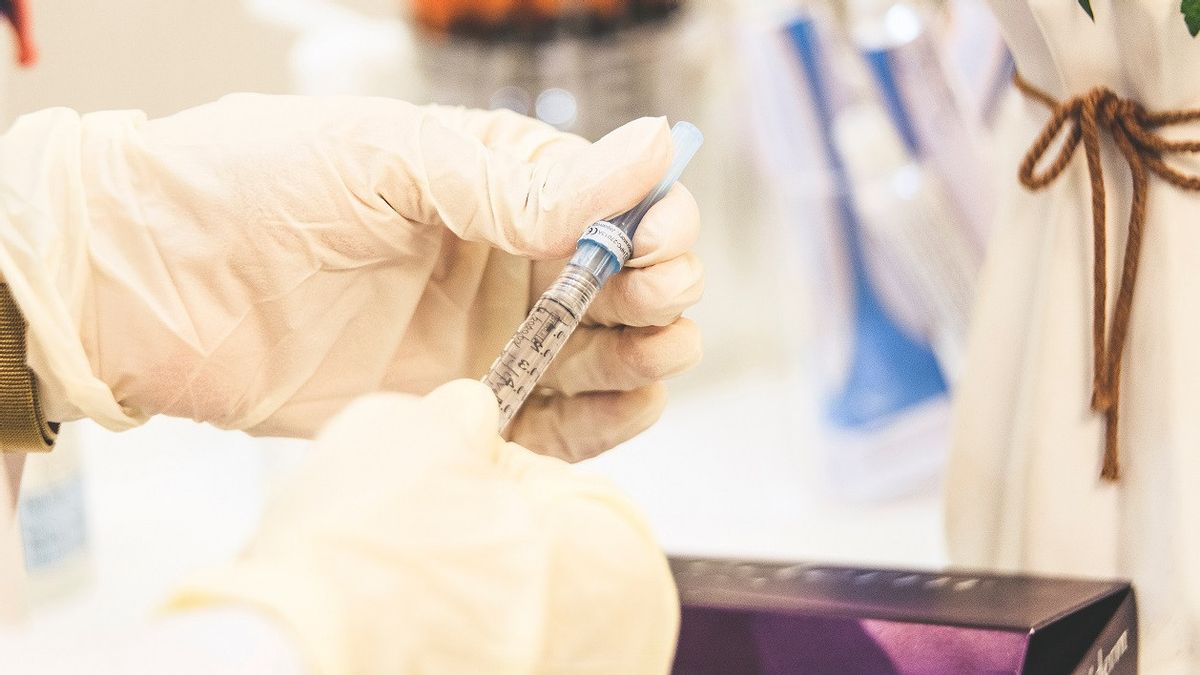
JAKARTA - The United States (US) government on Saturday local time authorized the single-dose Johnson & Johnson COVID-19 vaccine while preparing a global approval for use.
The US Food and Drug Administration (FDA) announced the authorization of emergency use of this vaccine for adults 18 years and over after an independent panel of experts unanimously approved its use last Friday.
United States President Joe Biden praised this move. However, he still warned Americans not to celebrate too soon.
"Things are still likely to get even worse as new variants spread," he said in a statement while urging people to continue to wash their hands, wear masks and maintain social distancing.
"There is a light at the end of the tunnel, but we cannot let our guard down now or assume that victory is inevitable," he added.
In a global trial of the Johnson & Johnson vaccine involving 44,000 people, the vaccine was found to be 66 percent effective at preventing moderate to severe COVID-19 four weeks after inoculation. And 100 percent effective in preventing hospitalizations and deaths from the virus.
There have been very few serious side effects reported in the trial, which also offers some preliminary evidence that the vaccine reduces asymptomatic infections.
However, further research is expected and the FDA last week rejected the idea of a vaccine preventing human-to-human transmission of COVID-19. However, there are no data yet to determine how long vaccine protection lasts.
The J&J vaccine is expected to be widely used worldwide because it can be shipped and stored at normal refrigerator temperatures, making distribution easier than the Pfizer Inc BioNTech SE and Moderna Inc vaccines, which are shipped frozen.
"This has the potential to play a very important role if we have sufficient doses because this is only a single dose vaccine and it will make it attractive to hard-to-reach people. One (dose) and done,” said an infectious disease expert at Vanderbilt University Medical Centers in Nashville Dr. William Schaffner.
The United States government, which has purchased 100 million doses of Johnson & Johnson vaccine, plans to distribute about 3 million to 4 million doses this week. That will be on top of the approximately 16 million doses of PfizerBioNTech and Moderna vaccines that are already planned to be shipped across the country.
"We are ready to launch," White House Senior Advisor Andy Slavitt wrote on Twitter after authorization. Johnson & Johnson plans to deliver a total of 20 million doses by the end of March.
The Johnson & Johnson vaccine is also under review by the European Union, with deliveries expected to start in April. Meanwhile, in South Africa, regulators are awaiting the FDA's decision, as their government will deploy more of the Johnson & Johnson vaccine against a variant of the virus called B.1.351 that is able to evade vaccine protection.
SEE ALSO:
To note, Johnson & Johnson said on Friday that the company is developing a second-generation vaccine that will target a South African variant. The first phase of the trial will begin next summer. Johnson & Johnson is also known to be testing a two-dose version of its vaccine, with results expected this summer.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)