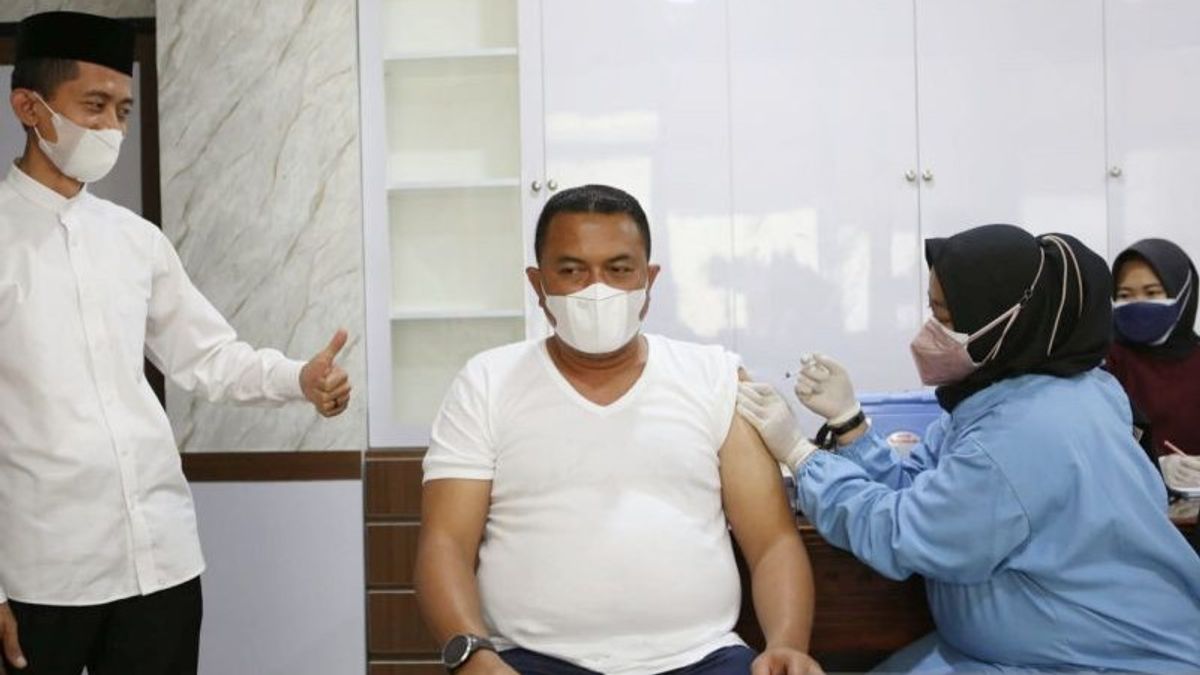
JAKARTA - PT Kimia Farma Tbk stated that the public can get the Sinopharm booster vaccine after obtaining Emergency Use Authorization (EUA) or emergency approval from the Food and Drug Supervisory Agency (BPOM) as an advanced dose vaccine or homologous booster for adults 18 years and over.
Thus, people who have received the complete primary dose of Sinopharm vaccine for at least six months can already receive the booster vaccine produced by the Beijing Bio-Institute Biological.
"The Sinopharm booster vaccine is here to help accelerate the vaccination program that has been launched by the government," said GM Corporate Secretary of PT. Kimia Farma Tbk in a statement in Jakarta, Saturday, February 12.
With the issuance of the EUA for the Sinopharm homologous booster vaccine, it means that it has added to the variant of the dosage regimen announced by the Ministry of Health and the POM Agency.
Change said, BPOM has evaluated the efficacy and safety aspects referring to the COVID-19 vaccine evaluation standard for the Sinopharm vaccine as a homologous booster dose for adults 18 years and over.
VOIR éGALEMENT:
"The Sinopharm vaccine as a booster is generally well-tolerated," said Change, quoted from ANTARA.
Meanwhile, the frequency, type, and severity of adverse reactions or adverse events (KTD) after booster administration were lower than during primary dosing.
Adverse events that often occur are local reactions such as pain at the injection site, swelling, and redness as well as systemic reactions such as headache, fatigue, and muscle aches, with a grade of 1-2 severity.
From the aspect of immunogenicity, the increase in humoral immune response for the measurement parameters of neutralizing and anti-IgG antibodies was 8.4 times and eight times, respectively, compared to before giving the booster.
In accordance with the results of the evaluation carried out by BPOM, the immune response after giving the booster was higher than the immune response produced during the primary vaccination.
The change added that people who have received the primary dose of Sinopharm COVID-19 vaccine through legal entities or business entities, as well as legal entities or business entities that wish to register their employees, can now register through portal.vaksingotongroyong.id or WhatsApp Biofarma at 0811 2060. 888.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)