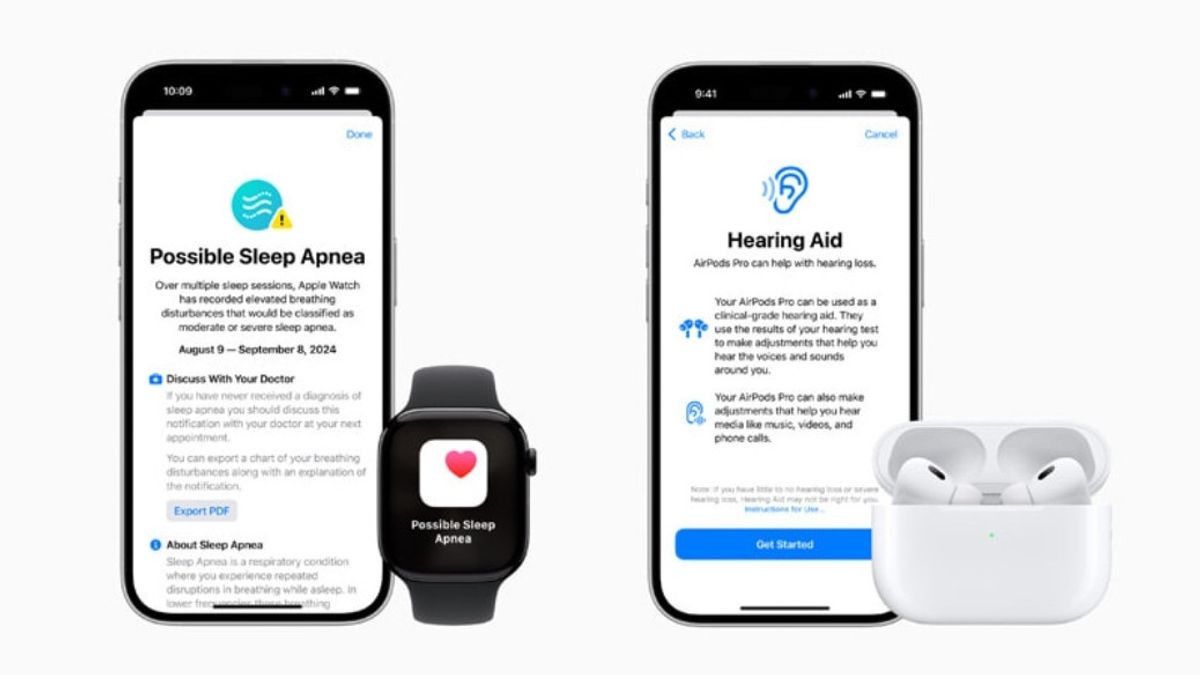
JAKARTA Sleep Apnea feature for Apple Watch has obtained permission from the US Food and Drug Administration (FDA) in early September. After receiving permission in the US, Sleep Apnea has now received permission from Canada.
Health Canada, the federal department regulating healthcare products in Canada, licenses the use of the Sleep Apnea feature in the country's compatible smartwatch. The license was issued on September 26.
The licensing from Canada is part of Apple's expansion pledge. The company had revealed that Sleep Apnea would be available in more than 150 countries and territories to support the health of Apple Watch users.
This sleep problem detection feature can only be found in the Apple Watch Series 9, Apple Watch Series 10, and Apple Watch Ultra 2. This feature uses device accelerometers to alert its users when they have sleep problems.
SEE ALSO:
Sleep apnea is a condition in which a person does not breathe several times while they are asleep. If not handled properly, sleep apnea can cause sudden heart attacks or stores that lead to death.
"If not treated, this condition can have serious health consequences over time, including increased risk of hypertension, type 2 diabetes, and heart problems," Apple said, quoted by 9to5mac.
Apple feels that this problem is quite important so they develop a metric of respiratory distress that can detect small movements related to normal respiratory pattern disorders. This problem will be analyzed every 30 days to conclude the symptoms of sleep apnea.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)