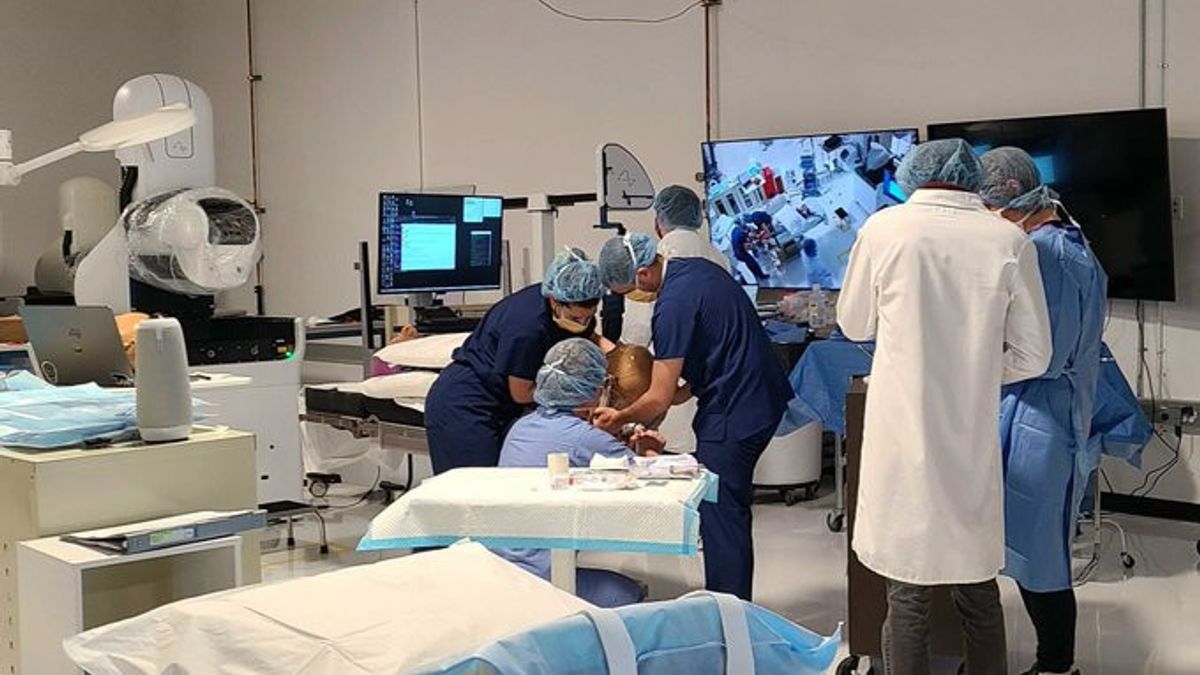
JAKARTA Neuralink, a technology company that couples chips to the brain, will list three patients into the study. These three patients will be tested for several years to evaluate the device. Based on a Reuters report, this research effort is seen in the details of the US government's clinical trial database. The study to be Neuralink is expected to be completed in 2026, while a more complete version will be completed in 2031. In the database, it is noted that the involved patients will be 22 to 75 years old with a quadriplegia condition or paralysis due to illness or brain injury. Patients examined must also suffer from quadriplegia for at least a year. Neuralink must use patients who have limited movements on the hands, wrists, or arms. The company may also examine patients who do not have any hand movements at all due to spinal cord injuries or abnormalities. This is not the first time Neuralink has attempted to conduct a study using human patients. Last year, the company owned by Elon Musk attempted to register ten patients to obtain clinical trial approval from US regulators.
SEE ALSO:
After obtaining clinical trial approval from the US Food and Drug Administration (FDA), Neuralink showed off his first patient in January. This patient named Noland Arbaugh suffered from paralysis on the shoulders down. After carrying out chip installation operations, Arbaugh can play video games and use the internet again. The patient can operate a computer because it moves the cursor using his mind.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)