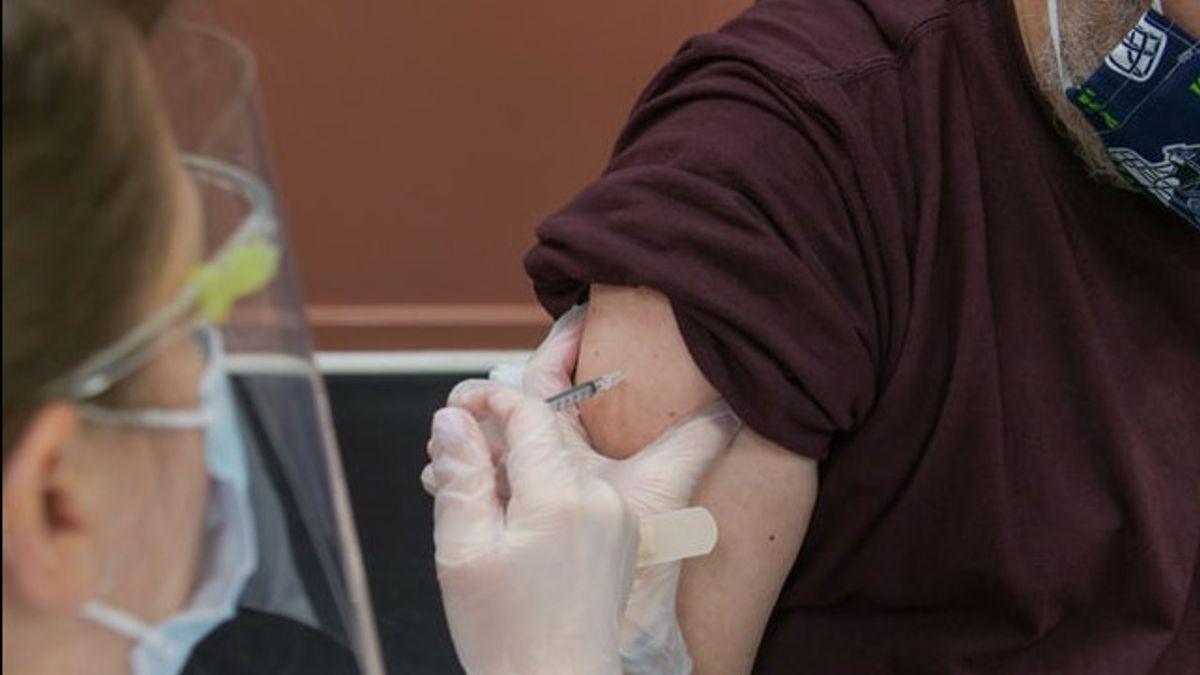
JAKARTA - PAN appreciates the government's decision to carry out vaccinations for children aged 6-11 years. This policy is considered very important along with the ongoing face-to-face learning (PTM) in schools.
"If it is PTM, the children will definitely mix with one another. Teachers and other administrative staff will also be part of the interaction and contact at school. This is what must be ensured that everything is safe. Well, one way is by implementing vaccination for students, teachers, and administrative staff at schools", said Saleh, Tuesday, November 2.
The member of Commission IX of the House of Representatives also asked the government to immediately start the vaccination. Saleh reminded, there are several steps that need to be taken by the government. First, ensure the availability of vaccines for children aged 6-11 years.
SEE ALSO:
Third, the government must immediately determine the mapping of the implementation of vaccination for these children. Because the procurement of vaccines is not simultaneous, said Saleh, it is necessary to determine the priority areas.
That way, according to the North Sumatran legislator, the implementation of this vaccination is in line with the task force program in breaking the chain of the spread of COVID-19.
"Vaccination for these children is considered easier. All you have to do is determine the schedule, then they are called in turn. There may be one or two who are afraid, but usually, they can be persuaded and encouraged", concluded Saleh.
"We can announce the issuance of permits for the use of the COVID-19 vaccine from the Sinovac Coronavac vaccine and the COVID-19 vaccine from Bio Farma for children aged 6 to 11 years", said Head of BPOM, Penny K Lukito, in a virtual press conference, Monday, 1 November.
Penny said this is good news because COVID-19 vaccinations for children are needed following the start of face-to-face learning at schools.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)