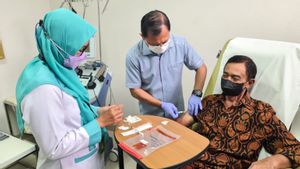
JAKARATA - Even though the COVID-19 pandemic has been sloping, efforts to fight the corona variant are still being carried out. In addition to the vaccine produced by various pharmaceutical companies, the Nusantara Vaccine initiated by Dr. Terawan Agus Putranto could be an alternative.
Some time ago the World Health Organization (WHO) suggested an update of the COVID-19 vaccine, in order to specifically target the mutated Corona variant due to the available COVID-19 vaccine being less effective in preventing infection and the risk of severe symptoms in COVID-19 patients.
This recommendation comes after previously, in March, WHO alluded to healthy children and adolescents may not need a COVID-19 vaccine injection.
The new recommendation was delivered by the WHO's Technical Advisory Group on Covid Vaccine Competition (TAG-CO-VAC) in a release at the Daily Mail UK, Friday, May 19, which recognizes and reiterates that the current COVID vaccine, including the virus-based (Wuhan), continues to provide substantial protection against severe illness and death.
"(However) a new formulation of the COVID vaccine is needed to increase protection against symptomatic diseases," wrote the Technical Advisory Group on Covid Vaccine Competition (TAG-CO-VAC) WHO.
They suggested that the COVID-19 vaccine be specially designed to fight the Omicron XBB subvariant which now dominates. The reason is, the XBB.1.16 variant, dubbed 'Arcturus', is known to be more infectious than others.
Previously, WHO had revealed the potential for Omicron XBB.1.16 subvariants to be more infectious than other existing Corona variants. As stated by WHO technical leadership for COVID-19, Maria Van Kerkhove, referring to laboratory research, XBB 1.16 has one additional mutation so it is more contagious and has the potential to be more pathogenic.
Responding to this, one of the founders of the Discussion Room, Dar Edi Yoga, hopes that the government can immediately provide regulations for the Nusantara Vaccine so that it can be mass produced, especially the Nusantara Vaccine made by Prof. Dr. Terawan has also been included in the international journal ClinicalTrial.gov adopted by WHO.
"The results of the Nusantara Vaccine clinical trial initiated by Lieutenant General TNI (Ret.) Prof. Dr. Dr. Terawan Agus Putranto, SpRad(K) have been published in an international medical journal in the journal Human Vaccines & Immunotheraetics which Scopus Q 1 indexed," said Yoga, Wednesday, May 31.
"The release titled A personal COVID-19 dendritic cell vaccine made at point-of-care: Feasibility, safety, and antigenspecific cellular immune responses was released on August 26, 2022," he added.
He said the results of clinical trials I and II of the Nusantara Vaccine were the first clinical trials to enter an international medical journal in collaboration with Indonesia and America. Previously, the development of the Nusantara Vaccine had also been published in the same journal with the title Dendritic cell vaccine as a potential strategy to end the Covid-19 pandemic. Why should it be Ex Vivo? which was released on May 26, 2022.
"The Nusantara vaccine has been proven to be able to prevent and treat various variants of COVID-19 and can only be injected once for a lifetime. This is based on various testimonies from volunteers who have received injections from Dr. Terawan," said Dar Edi Yoga who is also Deputy Chairman of the Central SMSI Advisory Council.
اقرأ أيضا:
The Nusantara vaccine is developed with a dendritic cell approach. The way it works, everyone will take blood samples to be explained by a vaccine kit formed from dendritic cells.
Furthermore, cells that already know antigen will be incubated for 3-7 days. The results will be injected back into the body. Inside the body, the dendritic cells are expected to trigger other immune cells to form a memory defense system against SARS CoV-2. That's the process carried out for the Nusantara Vaccine.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)