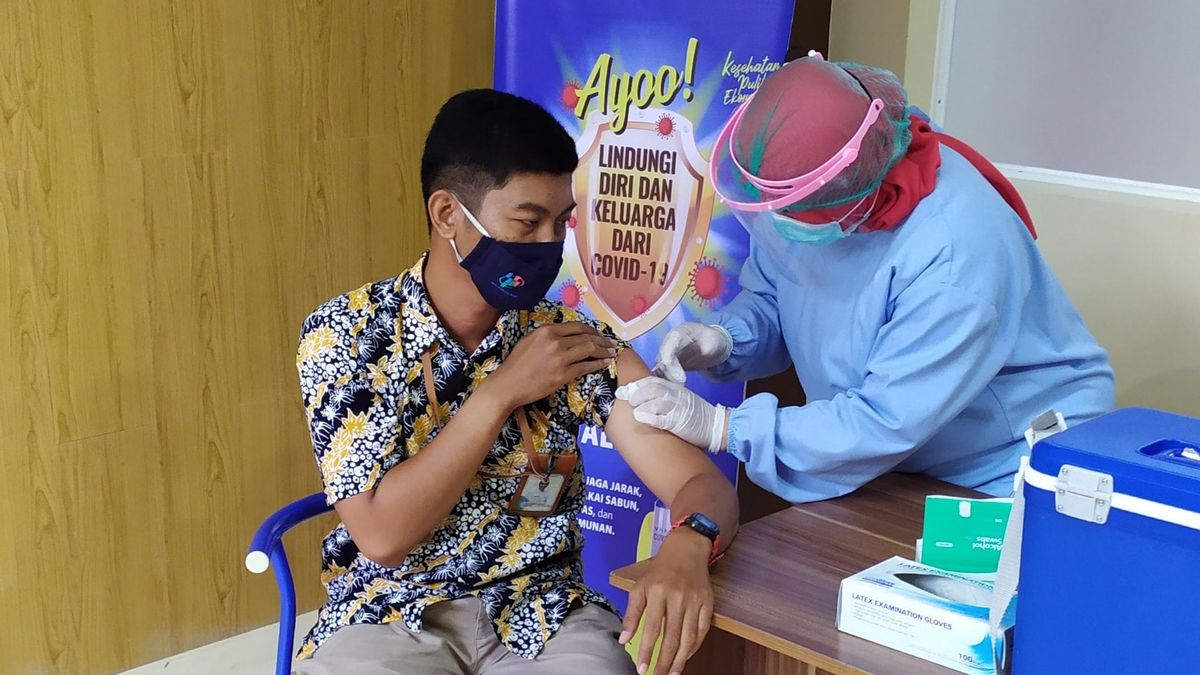
JAKARTA - Spokesperson for the COVID-19 Ministry of Health, Siti Nadia Tarmizi, appealed to the public not to be "picky" on the types of vaccines that the government is now circulating in the community, because the World Health Organization (WHO) has declared everything safe.
"So, there is no reason for the public to hesitant to join the vaccination program," he said during his presentation at the Productive Dialogue on Tuesday, May 18, as reported by Antara, Wednesday, May 18.
Siti Nadia said that to meet the vaccination needs of as many as 181 million target targets in Indonesia, around 426 million doses of vaccine are needed.
This, said Siti Nadia, would not be able to be fulfilled by just one vaccine manufacturer. In fact, countries in the world also have to compete fiercely in obtaining vaccine supplies for their population.
"At first Indonesia received the Sinovac vaccine, then at the end of March and early April 2021 we had the AstraZeneca vaccine, and in June or July 2021, other vaccines were scheduled to come, such as Novavax and Pfizer," he said.
Siti Nadia said the government would name all vaccines as COVID-19 vaccines so that they would no longer be based on producers.
SEE ALSO:
WHO, said Siti Nadia, stated that the AstraZeneca vaccine was safe and effective to protect people from the very serious risks of COVID-19, including death, hospitalization, and severe disease.
"We call all this the COVID-19 vaccine," he said.
Vaccines are currently in an urgent condition, where the government is trying to achieve herd immunity.
"People do not hesitate to join the vaccination program. The benefits of the vaccination program far outweigh the risks," Siti Nadia said.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)