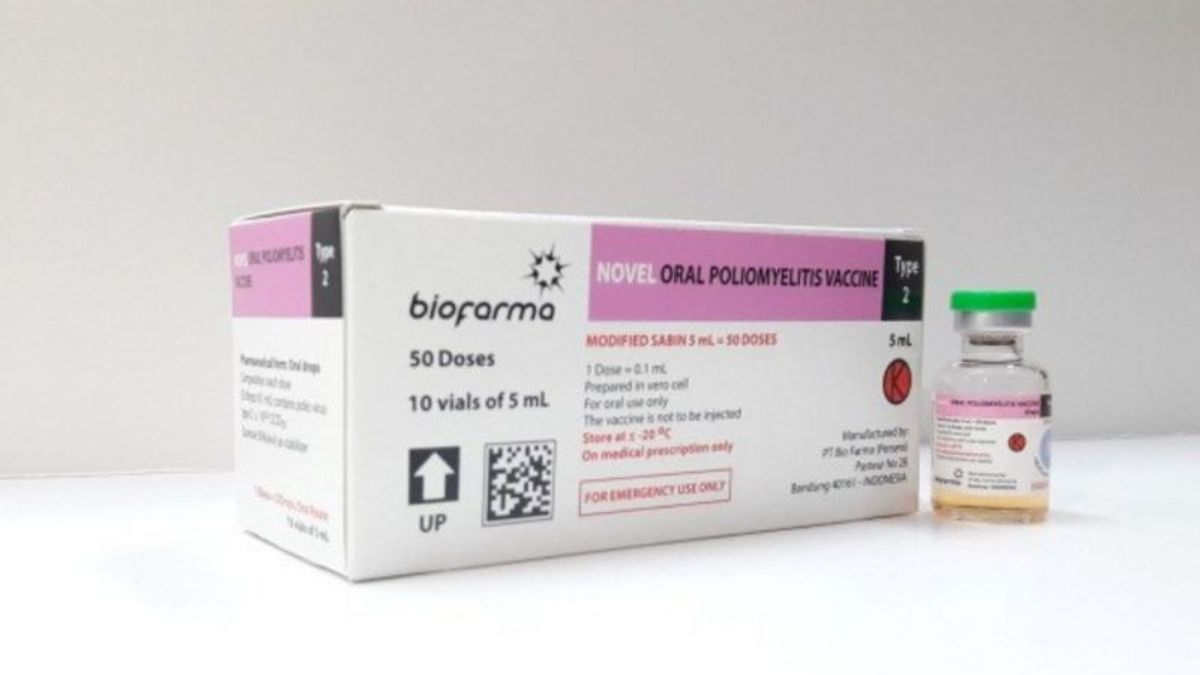
JAKARTA - The United Nations Children's Fund (UNICEF) has ordered a total of 200 million doses of the polio vaccine produced by PT Biofarma named the novel Oral Polio Vaccine type 2 (nOPV2).
"The number ordered by Unicef during 2020-2021 is 200 million doses of the nOPV2 vaccine," said Bio Farma's Head of Corporate Communication, Iwan Setiawan, confirmed by ANTARA from Jakarta, Monday.
Iwan said nOPV2 is the latest type of polio vaccine in the world which is intended for a number of countries that are still struggling with polio outbreaks.
The countries in question include those domiciled in the Eastern Mediterranean, Western Pacific, and several regions in Southeast Asia.
"One of them is Africa, which is still struggling with polio outbreaks, because there are still many countries in the region that have not been free from polio," he said.
SEE ALSO:
Iwan said the production of the vaccine was adjusted to the number of requests from Unicef when there were countries experiencing a spike in cases. Once the vaccine is ordered, Unicef will distribute it to a number of countries in need.
According to Iwan, the order for the vaccine made by Bio Farma was based on the WHO's approval for the use of the vaccine in an emergency (Emergency Use Listing/EUL) Polio vaccine on November 13, 2020.
Iwan said nOPV2 is a modified version of the existing type 2 monovalent OPV (mOPV2). In clinical trials, this nOPV2 provides the same protection against type 2 poliovirus with the advantage of being more genetically stable and having a lower chance of recurrence of polio cases from viral mutations in the vaccine.
The use of nOPV2 in this EUL condition, said Iwan, has been recommended by the Strategic Advisory Group of Experts (SAGE) on Immunization, an independent institution that recommends nOPV2 to overcome the Polio outbreak and also the Global Polio Eradication Initiative (GPEI) to ensure that during this EUL period, pay attention to safety standards.
"The world's progress against the polio epidemic has been 99.9 percent since the last 30 years, but this epidemic is difficult to eradicate," he said.
President Director of Bio Farma Honesti Basyir said prior to the issuance of the EUL from WHO, the Food and Drug Supervisory Agency (BPOM) had issued an Emergency Use Authorization Agreement limited to pandemic outbreak conditions for 50 doses of nOPV2.
Bio Farma is also conducting clinical trials of nOPV2 in several places, namely Belgium and Panama. Bio Farma is required to conduct a phase 3 clinical study to ensure the effectiveness and safety of the vaccine as an active poliomyelitis type 2 immunization.
In mid-October 2020, the POM Agency has awarded a certificate of Good Manufacturing Practice (GMP) for Building 43 at Bio Farma, as a production facility used for the manufacture of the nOPV2 vaccine.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)