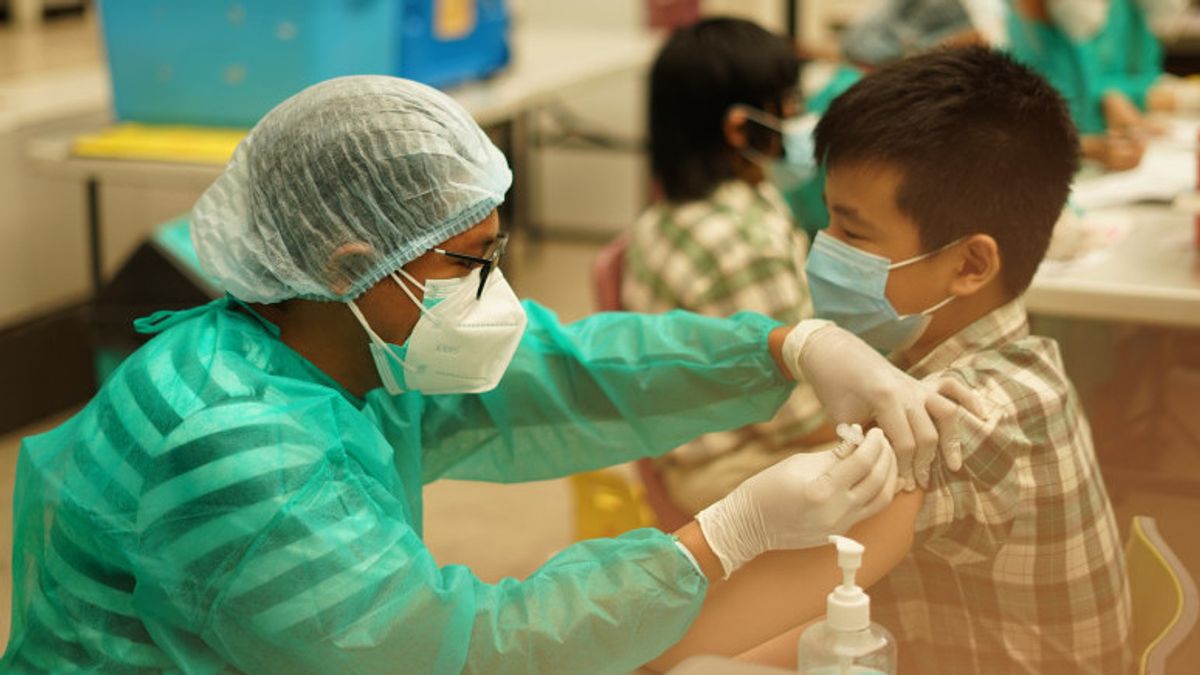
JAKARTA - This year's commemoration of National Children's Day (HAN) carries the theme "Protected Children, Advanced Indonesia" with the tagline "Children Care in a Pandemic Period". It's no exaggeration if HAN can be a moment for parents to register their children for COVID-19 vaccinations to protect themselves and their families.
The Indonesian Ministry of Health (Kemenkes) has issued Circular Letter Number HK.02.02/I/1727/2021 concerning Phase 3 Vaccination for Vulnerable Communities and the General Public and Vaccination of Children aged 12-17 Years.
The circular stated that in accordance with the intake from the National Immunization Expert Advisory Committee or the Indonesian Technical Advisory Group on Immunization (ITAGI) and the approval for the use of the COVID-19 Vaccine produced by PT Bio Farma (Sinovac) for the age group above or at 12 years from BPOM dated 27 June 2021.
The Food and Drug Supervisory Agency (BPOM) approved the use of the Sinovac COVID-19 vaccine, and this decision was taken based on a study conducted by the POM Agency together with the National Committee (Komnas) for Assessing the COVID-19 Vaccine, and the Indonesian Technical Advisory Group on Immunization (ITAGI), and the Indonesian Pediatrician Association (IDAI).

"After giving approval for the use of the COVID-19 vaccine for children aged 12-17 years produced by Sinovac/PT. Bio Farma, the POM will continue to monitor its use to guarantee the safety, efficacy, and quality of the vaccine after the vaccine is distributed," said the POM Agency. quoted from the official website.
How safe is the COVID-19 vaccine for children?
The Indonesian Pediatrician Association (IDAI) some time ago issued a number of recommendations regarding the provision of the COVID-19 vaccine to children and adolescents.
The first recommendation from IDAI, is the acceleration of COVID-19 vaccination in children using the inactivated COVID-19 vaccine made by Sinovac, because it is already available in Indonesia and there have been phase 1 and 2 clinical trials, the results of which are safe and high seroconversion.
Second, based on the precautionary principle, immunization should be started at the age of 12–17 years by considering the adequate number of clinical trial subjects, high mobility and the possibility of crowding outside the home, and being able to state AEFI complaints if any.
Further, a dose of 3 g (0.5 ml), an intramuscular injection in the deltoid muscle of the upper arm, was administered 2 times 1 month apart. For COVID-19 vaccination for children aged 3 -11 years, they are still waiting for the results of the study to assess safety and dosage with an adequate number of subjects.

The results of these considerations include the results of phase 1 and phase 2 clinical trials of Sinovac's CoronaVac vaccine in children aged 3-17 years using a randomized, double-blind, and placebo control method in Zanhuang, China.
In terms of safety, in phases 1 and 2 after 28 days of injection, Post-Immunization Adverse Events (AEFI) were found in 26-29 percent of the subject group, statistically not significantly different from the placebo group (24 percent).
The most AEFIs were mild and moderate pain at the injection site (13 percent). There was only one serious AEFI case, and there was no association with the vaccine.
Furthermore, AEFI in the age group 3 -11 years includes fever, while at the age of 12-17 years is mainly pain at the injection site, but there are no reports of fever.
Children can be infected and/or transmit the coronavirus from and to adults around them (parents, other people who live in the same house, people who come to the house, friends or teachers at school in face-to-face learning) even if they are asymptomatic. "To break the reciprocal transmission between adults and children, apart from strict health protocols, it is necessary to accelerate immunization for adults and children, especially adolescents with high mobility," said IDAI.
Parents can also protect their children by continuing to play and study at home, don't go to places where there are a lot of crowds, and continue to follow health protocols in a disciplined manner.
How to Access and Get Children's Vaccines
Through various information published by the government, there are a number of steps to get vaccines for children aged 12-17 years.
1. Vaccination participants must bring a family card or other document that includes the child's NIK
2. Passed the same screening, implementation, and observation mechanism as vaccination at >18 years old
3. Get permission from parents
4. Recording in the PCare application of vaccinations is included in the youth group
5. Bring pre-printed screens
6. Bring a pen
7. Vaccinate using Sinovac vaccine at a dose of 0.5 ml twice with a distance or interval of at least 28 days
Immunization recommendations during PPKM
IDAI has also issued recommendations regarding the implementation of immunization during the Community Activity Restrictions (PPKM) period which will last until the end of July.
"In order to support the implementation of the PPKM period, here are things that need to be understood together in the community. First, routine immunization in the Emergency PPKM area should be postponed for 3 weeks, starting on July 3, 2021. Immunizations for newborns are Hepatitis B and polio the first dose is still given," said IDAI through its official statement.
Furthermore, as long as immunization services in PPKM areas are postponed, parents should check the completeness of their child's immunization status in the KIA Book (maternal and child health books) and record immunizations that cannot be given during the PPKM period, then complete them immediately after conditions allow.
SEE ALSO:
Furthermore, COVID-19 vaccination services for ages 12-17 must continue according to the rules. Finally, the implementation of immunization services in areas that are not included in PPKM should continue with strict health protocols. "IDAI recommends that children not leave the house except for urgent matters such as serious health problems. During the PPKM period and after, strictly adhere to health protocols. Hopefully, we all survive this pandemic," he concluded as reported by Antara.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)