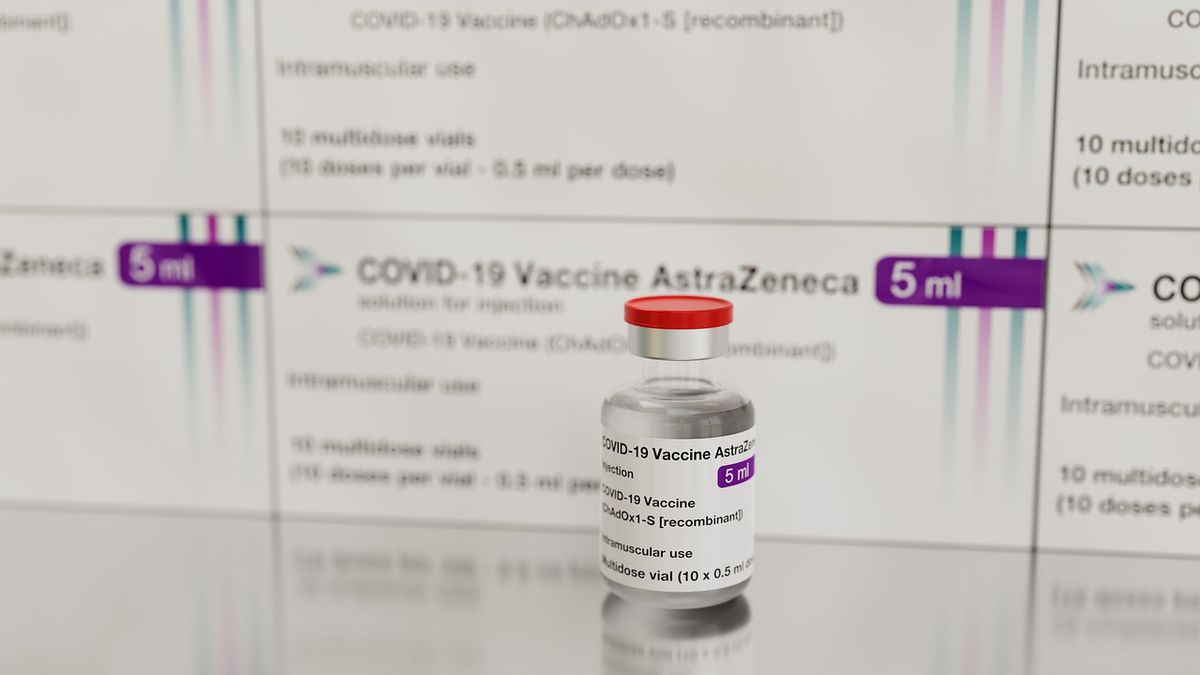
JAKARTA - Deputy Chairman of Commission IX of the DPR, Melki Laka Lena, stated that his party would summon the Indonesian Food and Drug Supervisory Agency (BPOM) because they allowed the AstraZeneca vaccine to be used in Indonesia. Following the suspension of the use of the AstraZeneca Vaccine by several countries, especially Europe, several incidents of post-immunization clotting (AEFI) were found.
"We have to call BPOM about the overall evaluation in Indonesia. Notes from Europe, plus a comprehensive report on the use of AstraZeneca in the country that we see two provinces in Bali and North Sulawesi (North Sulawesi) have an alarming incident. Later, we will check again with BPOM on this matter, "said Melki in his statement, Saturday, April 17.
According to him, BPOM must be responsible for the use of AstraZeneca Vaccine in Indonesia. BPOM's steps that still give permission to use AstraZeneca vaccines must be accompanied by strong arguments. The granting of permits still has to be done carefully even though the COVID-19 vaccine has high efficacy.
"Later we ask BPOM to be more careful in responding to the security aspects of AstraZeneca. Even though the efficacy is good, the security aspect must be the concern of BPOM, "explained the DPR member from the Golkar faction.
The Legislator for the East Nusa Tenggara II electoral district said that Commission IX of the DPR will first hold an internal meeting to determine the timing of the meeting with BPOM. If it is urgent, it is possible that a meeting with BPOM regarding the discussion of granting permits for the AstraZeneca Vaccine will take place during the current recess.
"We have indeed obtained permission from the leadership during the recess if there are important things to be discussed. "We will discuss it internally first to respond to the halted developments of AstraZeneca in various countries," said Melkiades Laka Lena.
Previously, BPOM RI stated that the AstraZeneca vaccine could still be used in Indonesia even though a number of countries in Europe began to suspend its use. The suspension of the vaccine made in England was carried out because AEFI was in the form of a blood clot.
"We conclude that injection with the AstraZeneca Vaccine can be continued. However, any incident should be taken into consideration, ”said the Head of BPOM Penny Kusumastuti Lukito through a video conference broadcast on the Youtube of the Indonesian POM Agency, Friday, April 16.
It is known, a number of countries began to suspend the use of the AstraZeneca Vaccine after several AEFIs were found to clot. One of the ones that recently stopped using the vaccine is Denmark.
The Ministry of Health stated that it is still waiting for a review from the National Immunization Expert Advisory Committee (ITAGI) and BPOM regarding the use of the AstraZeneca vaccine in Indonesia.
"Now we add a warning in the fact sheet statement, information to health workers who use Astrazeneca to be careful about the risk of the incident (blood clots, ed)," continued Penny.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)