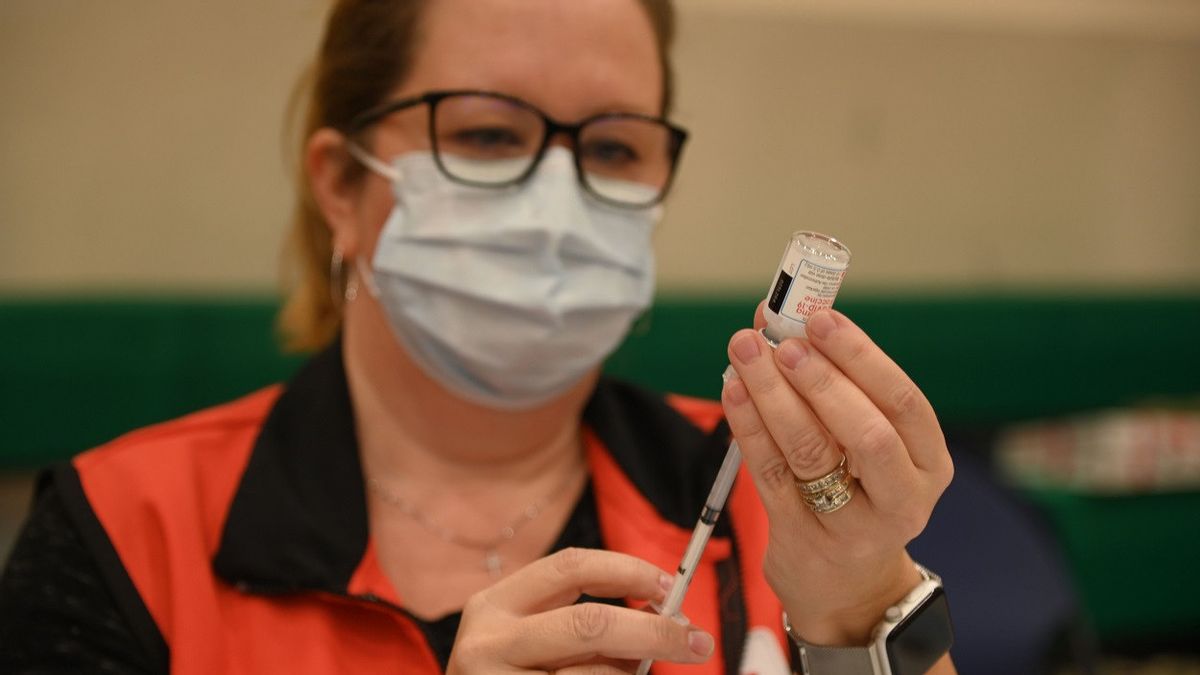
JAKARTA - Moderna Inc., said on Monday that a booster dose of its COVID-19 vaccine appears to protect against the fast-spreading Omicron variant in laboratory testing, that the current injectable version will continue to be the first line of defense against the Omicron variant.
Moderna said the decision to focus on its current vaccine, mRNA-1273, was driven in part by how quickly the newly discovered variant spread. The company still plans to develop a vaccine to protect against Omicron and hopes to start clinical trials early next year.
"What we have now is 1273," said Dr. Paul Burton, Moderna's chief medical officer, in an interview, citing Reuters December 20.
"It is very effective and very safe. I think it will protect people through the coming holiday period and during these winter months, when we will see the most severe stress from the Omicron variant."
Moderna further explained that two doses of the vaccine produced low neutralizing antibodies against the Omicron variant, but a booster dose of 50 micrograms increased neutralizing antibodies against the variant 37 times. A higher 100-microgram booster dose of the same vaccine prompted higher antibody levels, more than 80 times pre-boosted levels.

Moderna president Stephen Hoge said on a conference call the company does not currently plan to pursue approval for the higher dose.
The antibody levels produced by the low-dose injection were "quite above" levels signaling risk of 'breakthrough' infection for other variants of concern, Hoge said.
The data, which has not yet been peer-reviewed, tested blood from people who had received a vaccine against a pseudovirus engineered to resemble the Omicron variant. This is similar to data discussed last week by US infectious disease expert Anthony Fauci.
It may not be necessary to push antibody levels higher than that produced by a 50-microgram dose for many people, Hoge said. However, governments can opt for a higher dose version if they want to provide a better level of protection.
"Could higher be better? Of course. But do we have the data today to make a conclusive recommendation? No," Hoge said.
The company says doses of 100 micrograms are generally safe and well-tolerated, although there is a tendency for adverse reactions to occur slightly more frequently.
To note, the regulator of Uncle Sam's country authorized a 50 microgram booster for the Moderna vaccine in October. Previously, the first two injections of the Moderna vaccine were both 100 micrograms.
SEE ALSO:
Both the Moderna vaccine and the Pfizer/BioNTech vaccine have been associated with rare cases of heart inflammation, particularly in young men. Several studies have shown that the Moderna vaccine tends to cause heart inflammation at higher levels than the Pfizer vaccine.
The Omicron variant, a so-called highly contagious variant, was first detected last month in southern Africa and Hong Kong, has spread worldwide, and was reported in 89 countries, the World Health Organization (WHO) said on Saturday.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)