JAKARTA - The Drug and Food Control Agency (BPOM) has issued an emergency use authorization (EUA) or an emergency use permit for the COVID-19 vaccine produced by PT Bio Farma.
This EUA issuance is the second time after some time ago, the Drug and Food Control Agency also issued a similar license for a finished vaccine made by a Chinese pharmaceutical company, Sinovac.
Head of Drug and Food Control Agency, Penny Lukito said the issuance of the EUA was to support the acceleration of the national vaccination program held by the government. So far, she said, the implementation of vaccination in Indonesia has been going well.
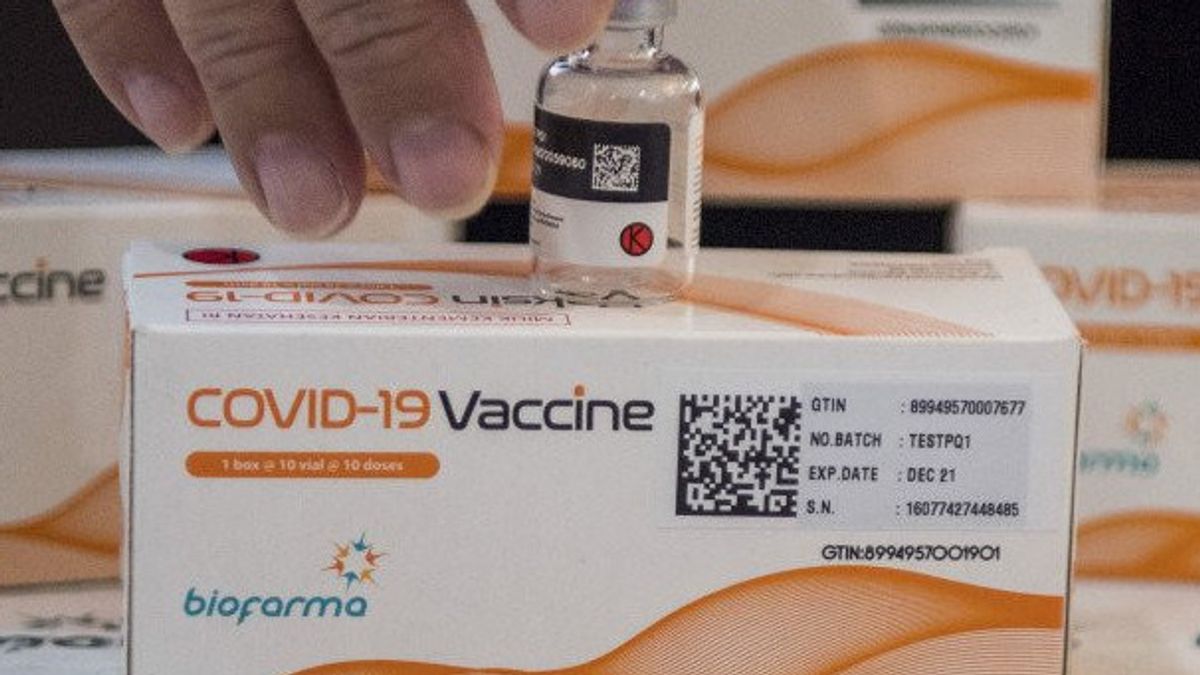
The vaccine produced by PT Bio Farma using raw materials from Sinovac is named COVID-19 Vaccine. This vaccine has a different packaging from the one previously distributed, namely CoronaVac. The EUA number for the vaccine is 2102907543A1.
"We appreciate and thank Bio Farma for following the timeline so that the EUA for vaccines produced by Bio Farma can be approved today", she said, in a virtual press conference, Tuesday, February 16.
Penny explained that this vaccine has a 5 ml vial supply containing 10 doses of vaccine per vial, which is an inactivated virus. This vaccine is packaged in 10 vials per box so that in 1 box there are 100 doses. The temperature is 2 to 8 degrees Celsius.
Furthermore, Penny said, each vaccine vial was equipped with a 2D barcode containing the vaccine's identity so that it could not be faked.
"Giving out the EUA to this vaccine is separate due to differences in the place of production and packaging", she said.
As is known, PT Bio Farma (Persero) as the vaccination organizer and the party producing the vaccine has provided 13 million COVID-19 vaccines as of 11 February 2021 yesterday. The 13 million vaccines are a total of the 13 batches produced by the company.
The vaccination process is carried out in stages throughout Indonesia to reduce the spread of COVID-19. This is because the Food and Drug Control Agency and the government continue to prioritize safety aspects of the quality and efficacy of the vaccines used in the national vaccination program.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)