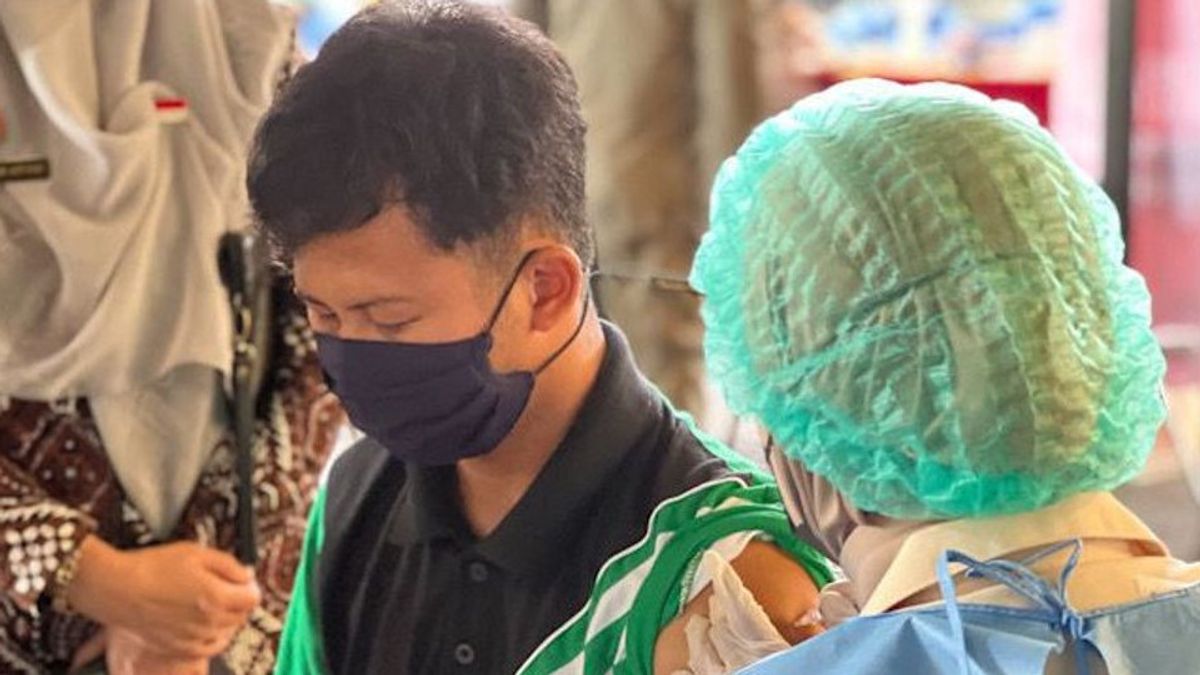
JAKARTA - Head of the National Research and Innovation Agency (BRIN) Laksana Tri Handoko is pleased with the success of phase 1 clinical trial of the Red and White vaccine whose vaccine seeds were developed by Universitas Airlangga (Unair). For BRIN, this is a big leap in developing vaccines independently in Indonesia.
"This is a big leap, not only for the Unair team, but also for the six other Red and White vaccine teams," said Laksana Tri Handoko, quoted from Antara, Wednesday, February 16.
The implementation of phase 1 clinical trial of the Red and White Unair vaccine has also attracted the attention of the entire Red and White vaccine development team. The reason is that it will provide learning for the entire team when they also enter the same phase.
He appreciated the efforts of all parties involved in bringing the Red and White Unair vaccine to the phase 1 clinical trial stage.
"For the Red and White vaccine, we are very grateful that finally there is a team that has successfully entered the phase 1 clinical trial phase," he said.
In addition to Universitas Airlangga, there are six other teams that have participated in developing the Red and White vaccine, and all of them are members of the national consortium for the development of the Red and White vaccine.
The teams in the national consortium for the development of the Red and White vaccine are Airlangga University, University of Indonesia, Bandung Institute of Technology, Gadjah Mada University, Padjadjaran University, the former Indonesian Institute of Sciences (LIPI), and the Eijkman Institute for Molecular Biology.
Each team developed the Merah Putih vaccine using a different method, ranging from a vaccine based on virus inactivation to a vaccine based on recombinant protein.
However, the fastest progress has come from Universitas Airlangga, in collaboration with PT Biotis Pharmaceuticals Indonesia.
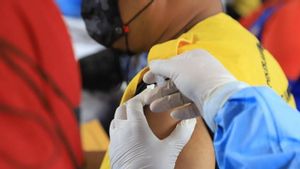
Currently, the Merah Putih vaccine whose vaccine seeds were developed by Universitas Airlangga is undergoing a phase 1 clinical trial process by injecting the vaccine to 90 volunteers aged at least 18 years.
Meanwhile, other teams are still in the optimization stage of antigen yield, and some are conducting preclinical tests.
SEE ALSO:
The Ministry of Religion's Halal Product Assurance Administration Agency (BPJPH) will immediately issue a halal certificate for the Red and White vaccine after going through a product audit at LPPOM MUI and the determination of halal products by the Indonesian Ulema Council (MUI) Fatwa Commission.
"The Ministry of Religion's BPJPH will immediately issue a halal certificate following the issuance of the MUI halal determination for the Red and White vaccine produced by PT Biotis Pharmaceuticals Indonesia," said Head of the Ministry of Religion's BPJPH, Aqil Irham.
According to him, the issuance of a halal certificate is the end of the halal certification process. Based on Law 33/2014 and PP 39/2021 halal certificates are issued by BPJPH, after going through a number of stages, including product audits by the Halal Inspection Agency (LPH) and the determination of halal products by the MUI Fatwa Commission.
The Red and White Vaccine has been determined to be halal through the MUI fatwa session on February 7, 2022. Meanwhile, LPPOM MUI as LPH has conducted an audit of the Red and White vaccine on January 14.
"So, MUI issues halal regulations, BPJPH issues halal certificates," he said.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)