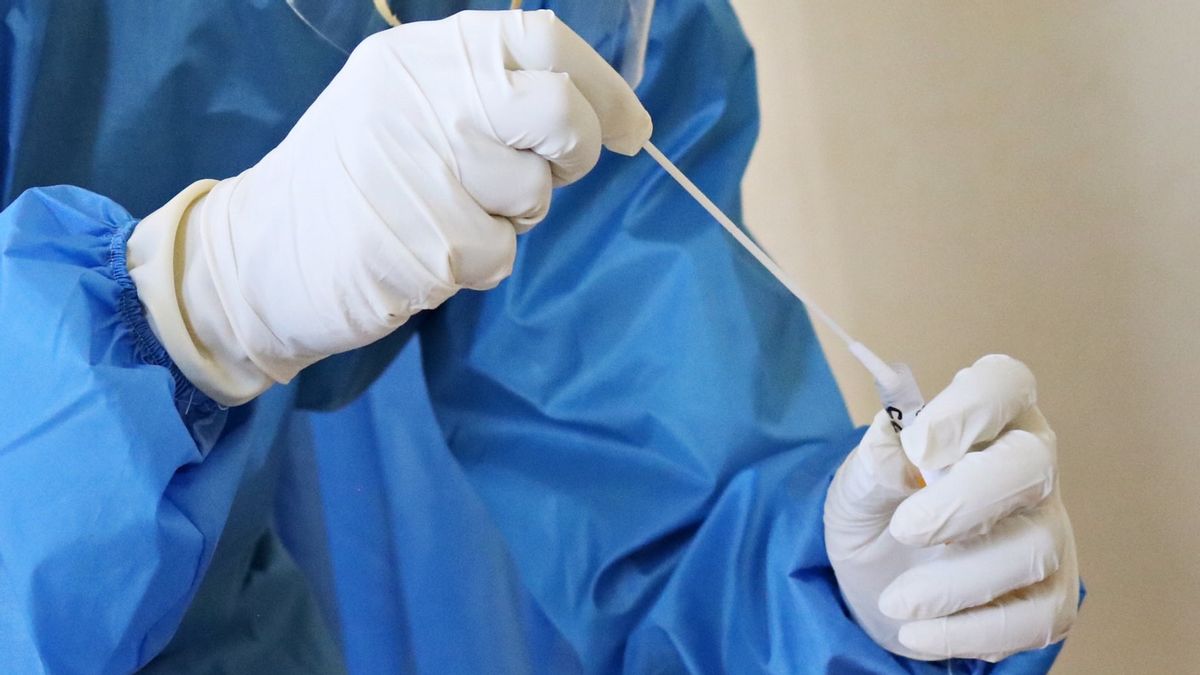
JAKARTA - PT. Kalbe Farma Tbk officially launched a COVID-19 test using a saliva or saliva sample called InnoLAMP. The COVID-19 test made by these nation's children can also be used as a condition for traveling within the country.
Director of PT. Innolab Sains Internasional (KALGen Innolab), Henry Sukardi, said that the COVID-19 test with saliva samples using the RT-LAMP method can specifically detect nucleic acids which are genetic material of the SARS CoV-2 virus. The results were similar to those with RT-PCR.
Furthermore, Henry said, RT-LAMP is a molecular test that is included in the category of Nucleic Acid Amplification Test (NAAT) together with RT-PCR and TCM according to the Decree of the Minister of Health of the Republic of Indonesia Number HK. 01.07/MENKES/446/2021. So, it can be used as a travel requirement.
"Because this has been included in KMK 446. For (conditions) travel within the country or in Indonesia, it can be used. For abroad, each country has its own policies. So, further policies must be seen," he said at the virtual conference press, Friday, March 19.
Henry said the advantage of using the COVID-19 saliva test is convenience. Because, it can be done independently and there is no need for special sampling techniques. In contrast to the RT-PCR which takes the sample through the nose.
Then, he said, the results of this test had an accuracy equivalent to that of RT-PCR. Furthermore, the results obtained are also very fast, only takes about 2 hours. To carry out this saliva COVID-19 test, the public must spend IDR 488,000.
However, Henry said, there is a special price until March 31, 2021, where people only need to pay IDR 400,000.
"Compared to the price of PCR, especially for the same day service, this price is very much more efficient (only IDR 400,000)," he said.
Henry said this test kit can be used from children to adults. However, Henry suggested that those wishing to use this tool should fast for 30 minutes in order to get more accurate results.
SEE ALSO:
"Who can use it? As long as the patients are able to produce saliva, they can be examined," he said.
On the same occasion, IVD Division Research Manager for Stem Cell and Cancer Institute, Akterono D. Budiyati, said the presence of this innovative test could be a very good choice because it has high accuracy performance, with 94 percent sensitivity and 98 percent specificity.
Based on a study from the Yale School of Public Health, Akte said, the level of the COVID-19 virus in saliva is comparable to a nose or mouth swab. It was also conveyed that saliva specimens from 20 COVID-19 positive individuals could be stored at room temperature or 19 degrees Celsius for up to 5 days.
"The saliva sample does not require special tools and does not pose a risk of vomiting or a sensitive nose, which makes it very easy for children, hypersensitive people, and is the right choice when fasting is approaching," he said.
Furthermore, Akte said, his party was conducting research on this COVID-19 test with saliva starting mid-2020. The test kits were able to distinguish individuals infected with SARS-CoV-2 from those who did not experience an infection.
This innovation certainly strengthens the company owned by conglomerate Boenjamin Setiawan as the leading and largest private pharmaceutical company in the country. Boenjamin Setiawan himself is the 8th richest person in Indonesia who founded Kalbe Farma since 1966.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)