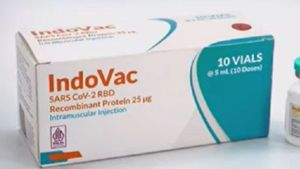
JAKARTA - PT Bio Farma (Persero) has obtained an Emergency Use Authorization (EUA) approval or an emergency use permit from the Food and Drug Supervisory Agency (BPOM) regarding the use of the IndoVac vaccine as a further vaccine or booster (Booster) for ages 18 years and over.
The Emergency Use Authorization (EUA) approval or emergency use permit was obtained after BPOM issued a letter dated April 21, 2023 regarding changes to the drug INDOVAC.
President Director of Bio Farma, Honesti Basyir said that the acquisition of EUA from BPOM added to the list of uses of the IndoVac vaccine as a booster for ages 18 and over.
Honesti said, previously Bio Farma had received the EUA for the IndoVac vaccine as a booster for people aged 18 years and over who had received primary vaccines from Sinovac and Astra Zeneca. Most recently, Bio Farma has just managed to get EUA for the IndoVac vaccine for the COVID-19 primary vaccine booster from Pfizer.
"This proves that the IndoVac vaccine, made by the nation's children, has the same quality as global products," Honesti said in an official statement, Tuesday, May 2.
Honesti said that IndoVac does not only fulfill the elements of safety, quality and efficacy but has more value, namely that it has officially obtained Fatwa and Halal Decree from MUI which then pocketed a halal certificate from the Halal Product Guarantee Agency (BPJPH).
So that the products can be accepted by all the world's population. Halal certification is one of IndoVac's advantages in the global market," he said.
Honesti said the results of the Booster Clinical Trial of Primary Heterolog Sinovac, AztraZeneca and Pfizer stated that the tubsin was safe. The most post-immunization follow-up events (AEFI) are pain at the injection site and muscle aches that are mild.
The IndoVac vaccine as a heterologous booster dose of the Primary Vaccine (Sinovac, AztraZeneca and Pfizer) can increase antibody titer and neutralizing titer against the Omicron variant. There is a significant increase in antibody titers after the booster dose compared to the baseline.
"Based on IndoVac's booster clinical trial data, it can be concluded that the IndoVac vaccine has good immunogenicity and safety profiles as a booster dose of participants who previously received primary doses of Sinovac, AstraZeneca, and Pfizer vaccines," he explained.
Honesti said the COVID-19 pandemic was still not over, based on data from the covid19.go.id site, as of May 1, 2023, there were 13,880 active cases of COVID-19.
SEE ALSO:
The public, he continued, still has to remain vigilant by maintaining prokes and vaccinations, especially booster vaccines based on data from the Ministry of Health, relatively many residents have not been vaccinated against boosters.
"Bio Farma continues to support the government's efforts to strengthen health infrastructure, one of which is through the domestic production booster vaccination program, namely IndoVac," he said.
IndoVac is a COVID-19 vaccine based on protein recombinant subunit technology which is used as an active immunization of COVID-19 caused by the SARS-CoV-2 virus. IndoVac has also obtained halal and MUI fatwas and halal certificates from BPJPH, Ministry of Religion and is a domestic product made by the nation's children with a domestic content level (TKDN) reaching 89.84 percent.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)