JAKARTA - The state-owned pharmaceutical company PT Indofarma Tbk seems to have had an anomaly from the COVID-19 pandemic that hit Indonesia. In the midst of this national disaster, Indofarma got 'blessings' from the health business that it is doing.
One of the blessings, namely the demand for medical devices (medical equipment) to Indofarma has increased. Finance Director of Indofarma Herry Triyatno said that the increase in orders for medical devices mainly occurred in the first half of this year. He said the orders came from private companies and state-owned companies.
Indofarma will also become a distributor of the corona virus vaccine or COVID-19 which will be produced by the holding company of BUMN Pharmaceuticals, PT Bio Farma (Persero), which is working with Sinovac Biotech Ltd to develop a virus vaccine that has spread throughout the world.
President Director of Indofarma, Arief Pramuhanto, said that the plan was for the third phase of clinical trials to be carried out by the holding of the BUMN Pharmacy holding in July 2020. Arief said that Indofarma would later cooperate with PT Kimia Farma Tbk in distributing vaccines.
"Distribution will be carried out after Bio Farma has entered the mass production stage of the COVID-19 vaccine, which is estimated to be in March or May 2021," Arief told VOI, Tuesday, July 21.
With these various positive achievements, Indofarma is optimistic that it will pocket IDR1.645 trillion in revenue by the end of 2020 with IDR 30 billion in profit.
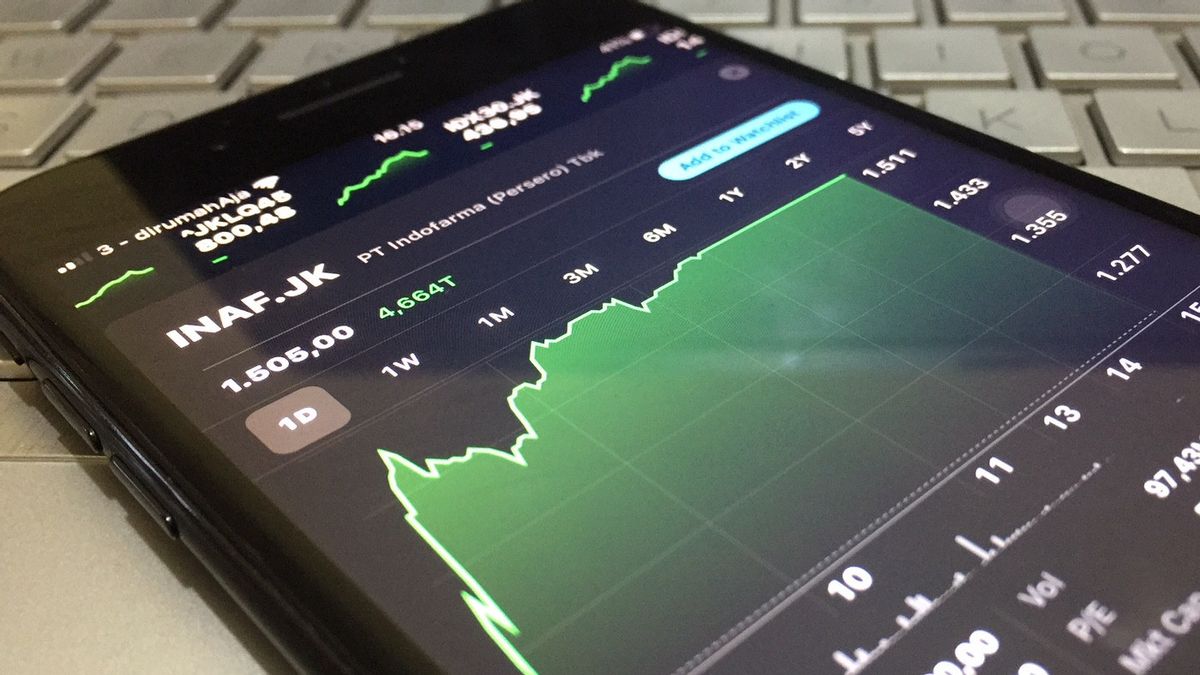
The shares of the issuer coded INAF also jumped quite significantly and became the stock with the highest gain today. Indofarma's shares at the close of Tuesday, July 20, were recorded to have strengthened 24.90 percent or 300 points compared to the previous day's close, to a level of IDR1,505.

CSA Research Institute Senior Analyst Reza Priyambada told VOI that market players responded positively to the news about the progress of the COVID-19 vaccine, which will be distributed by Indofarma when it is in Indonesia.
However, continued Reza, when the vaccine has arrived in Indonesia, it must be tested first and certified halal. According to him, there are still a number of processes until the vaccine is really ready for distribution and consumption.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)