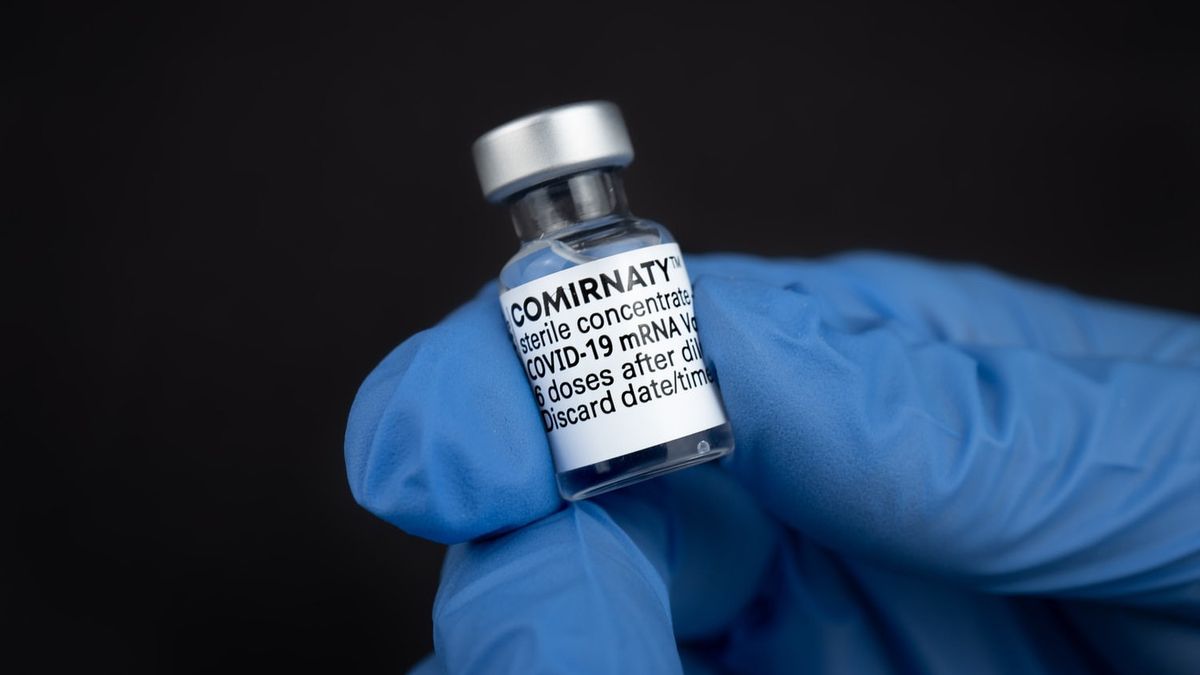
JAKARTA - The Food and Drug Administration (BPOM) has issued an emergency use authorization (EUA) for the Pfizer vaccine from the United States.
In general, BPOM said that this COVID-19 vaccine could be tolerated by all age groups. However, there are still side effects after the injection.
"The most common incidents include pain at the injection site, fatigue, headache, muscle aches, joint pain, and fever," said Head of BPOM Penny K Lukito in a press conference broadcast on BPOM RI YouTube, Thursday, July 15.
Furthermore, he said the efficacy of the COVID-19 vaccine with this mRNA platform had an efficacy of 95.5 percent for the age group of 16 years and over. Meanwhile, for adolescents aged 12-15 years, the efficacy reaches 100 percent.
The vaccine is administered at a dose of 0.3 ml and the injection is carried out in two doses at an interval of three weeks.
For information, there are currently six COVID-19 vaccines that have received EUA from BPOM to be used in Indonesia. Besides Pfizer, BPOM has issued EUA Coronavac from Sinovac from China, AstraZeneca obtained from COVAX facilities, Sinopharm from Beijing, and Moderena from the United States.
BPOM said the Pfizer vaccine uses an mRNA platform that has special storage specifications, namely using ultra low temperatures. The temperature should be in the range of -90 to -60 degrees Celsius.
Previously, Health Minister Budi Gunadi Sadikin said that 50 million US-made Pfizer vaccines would soon arrive in Indonesia.
This was agreed in the Ministry of Health's collaboration with Pfizer-BioNTech in the provision of a vaccine called 162b2 throughout 2021.
Minister of Health Budi said he welcomed the cooperation in the procurement of Pfizer vaccines in Indonesia. The vaccine is one of the COVID-19 vaccines used for the accelerated vaccination program in Indonesia.
"I thank you for your cooperation in helping to meet the needs of the COVID-19 vaccine in Indonesia. With the increase in the stock of 50 million doses of the Pfizer brand of vaccine, it is hoped that it will accelerate the implementation of vaccinations in Indonesia," said Budi on Wednesday, July 14.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)