Elon Musk's Neuralink has just stated that it has received approval from the Food and Drug Administration (FDA), United States (US), to launch its first human clinical study.
If this information is valid, it means that soon humans can have devices from the brain-computer interface company, which are implanted in their heads.
"We are very pleased to share that we have received the FDA's approval to launch our first human clinical study!", Neuralink said via its official Twitter.
We are excited to share that we have received the FDA’s approval to launch our first-in-human clinical study!This is the result of incredible work by the Neuralink team in close collaboration with the FDA and represents an important first step that will one day allow our…
— Neuralink (@neuralink) May 25, 2023
We are excited to share that we have received the FDA's approval to launch our first-in-human clinical study! This is the result of extraordinary work by the Neuralink team in close collaboration with the FDA and representatives an import first step that will one day allow our...
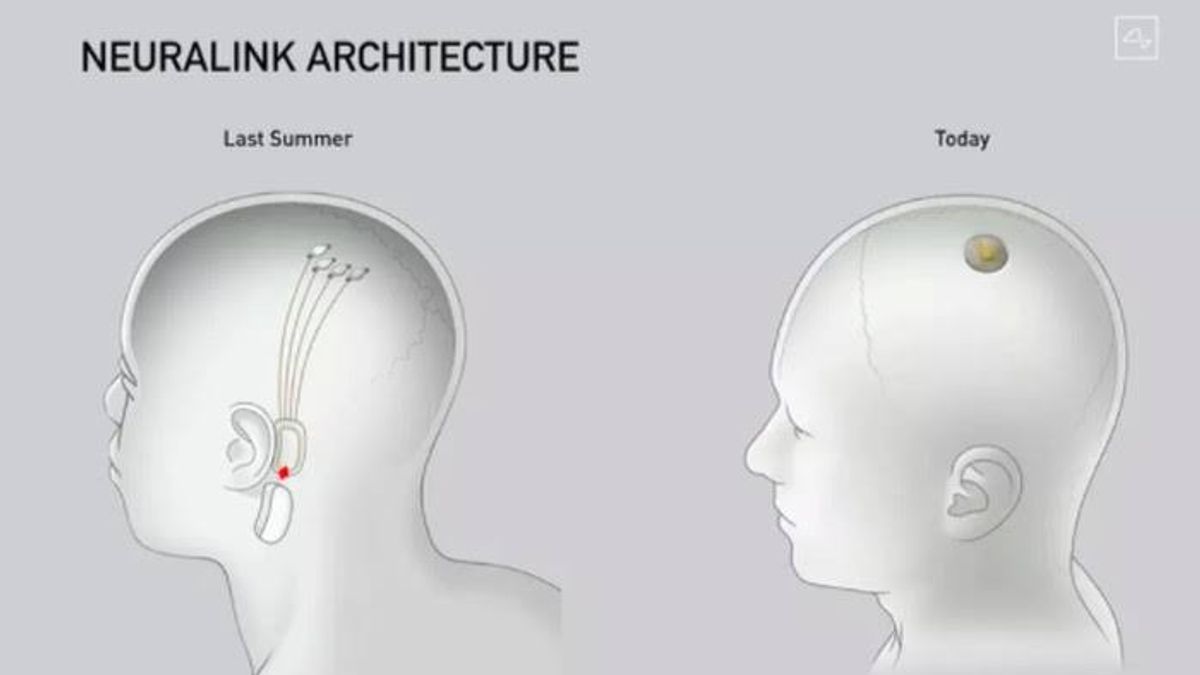
Currently, Neuralink is building a brain implant called Link to help people with severe paralysis can communicate again only with external control technology that uses neural signals.
This means that people suffering from severe degenerative diseases such as ALS can eventually regain their ability to communicate. They just move the cursor and type with their thoughts.
"This is the result of extraordinary work by the Neuralink team working closely with the FDA and is the first important step that will one day allow us," Neuralink explained.
Neuralink itself is a newly emerging brain-computer interface industry, or BCI. Where BCI is a system that outlines brain signals and translates them into commands for external technology.
Scientists have long developed BCI technology for decades, but accepting FDA approval for commercial medical devices is not a small task.
Where the company must succeed in conducting several rounds of testing and very comprehensive data security collection. However, Neuralink has already received the green light from the FDA to conduct human studies. That means the company is one step ahead to try out the market.
Even so, Neuralink has recently been accused of abusing its monkey test subject, but it has been denied by the company, and is being investigated for allegedly transporting contaminated devices removed from monkeys.
The FDA has also rejected the early 2022 Neuralink app for human trials, but appears to be getting dozens of issues that the company needs to deal with.
Unfortunately, Neuralink will not be the first to invest brain-computer interfaces in humans. Synchron was approved by the FDA to start trials in the US in 2021.
Synchron announced the first brain-computer implants in the US in July. Earlier this year, the company also published the results of previous research on four human patients in Australia. This was quoted from CNBC International and The Verge, Friday, May 26.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)