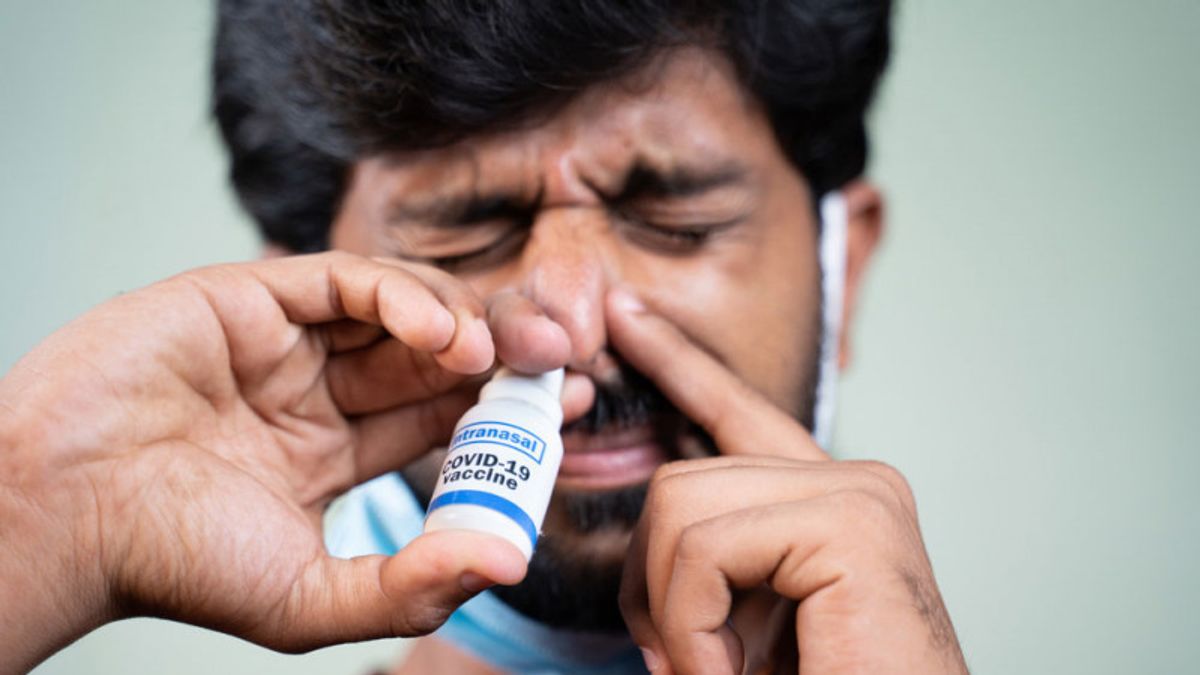
JAKARTA - Two nasal spray model COVID-19 vaccines under development in Thailand will begin human trials in late 2021, after trials on mice showed promising results, government officials said on Wednesday local time.
Developed by the National Center for Genetic Engineering and Biotechnology, the vaccine is based on adenovirus and influenza, said deputy government spokeswoman Ratchada Thanadirek.
Ratchada explained, after trials on mice, the first phase of human trials will begin later this year, awaiting approval from drug and food regulators.
"The experiment will also test the protection against the Delta variant," said Ratchada as quoted by Antara, Wednesday, August 11.
"Phase two is scheduled for March 2022 and a wider production target in mid-2022, if the results are good," he continued.
Please note that countries around the world are developing nasal spray vaccines to help prevent and treat COVID-19. The nasal lining was identified as the main entry point for the virus.
Thailand's other domestic vaccines, an mRNA vaccine made by Chulalongkorn University and an inactivated virus developed by Mahidol University, are also scheduled to begin phase two of human trials this August.
Thailand's vaccination program has so far relied on China's Sinovac, AstraZeneca and Sinopharm vaccines. Meanwhile, the Pfizer/BioNTech vaccine is being administered as a booster dose to front-line medical personnel receiving two doses of Sinovac.
"A total of 32.5 million doses of Pfizer/BioNTech vaccine will arrive this year, consisting of 30 million doses ordered and vaccine grants from the United States," said Health Minister Anutin Charnvirakul.
To date, about 6.8 percent of Thailand's 66 million-plus population have received two doses of the COVID-19 vaccine.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)