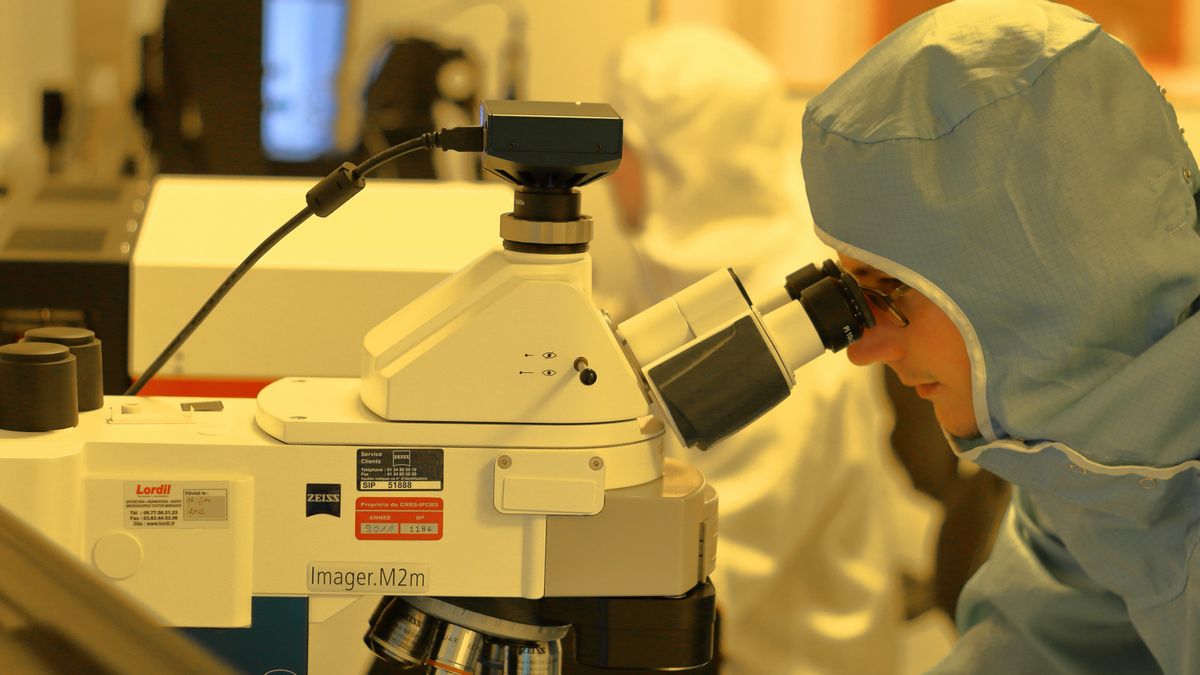
JAKARTA - Johnson & Johnson is one of the companies that is seriously working on the COVID-19 vaccine candidate. With a vaccine candidate labeled JNJ-78436735, Johnson & Johnson has entered phase 3 or ENSEMBLE clinical trials involving 45,000 participants.
Johnson & Johnson Global Head of Janssen Research & Development Mathai Mammen launched the official Johnson & Johnson website, saying that his party continues to strive to develop vaccines through a combination of experience with collaboration and partnerships with governments, authorities and the scientific community around the world
"From March to June we experimented with various candidates. Before finally we decided to become two candidates in June and only one candidate in July," explained Mammen.
"We then combined the two phases of traditional research into a single phase known as Phase 1 / 2a, to check safety and immune response in just a few months. After having strong data, we switched to Phase 3 clinical trials with selected doses and more. participants, "he explained.

Global Head of Janssen Research & Development Johnson & Johnson Mathai Mammen. (Source: johnson & johnson)
He further explained that in developing vaccines, his party also took into account the representation of all races, ethnicities, ages and medical backgrounds in the clinical trials that were carried out.
The important target of the ENSEMBLE study that was conducted was to achieve complete immunity by the 28th day. And, while looking at the fastest development of protection on the 14th day.
Regarding how the vaccine made by Johnson & Johnson, Mammen revealed that the vaccine they made was different from the mRNA type vaccine.
If mRNA makes proteins to trigger an immune response in the body. Johnson & Johnson's vaccine uses adenovirus (a type of virus that causes the common cold and is made unable to replicate).

"Adenovirus carries the gene from the corona virus into human cells, which then produces the corona virus spike protein, but not the corona virus itself. This spike protein is the prima donna of the immune system to fight further infection by the virus," he said.
"Adenovirus is well known in the human population. Previously we have used it in the Ebola vaccine and investigative vaccines for HIV and RSV. So we have a lot of safety data. And, the temperature of the vaccine will be more stable," he said.
Mammen added that his party needs to ensure that all aspects of the COVID-19 vaccine development program carried out meet safety and quality standards. All production and distribution processes comply with all applicable regulations.
This includes raw materials and components covering procedures, manufacturing facilities, testing laboratories, including analytical equipment and methods, shipping and routes, and shelf life and stability.
"We hope that this achievement can be achieved in February, so we can consider applying for our vaccine Emergency Use Authorization (EUA) with the US Food and Drug Administration (FDA), as well as filing regulations in other countries," he concluded.
It is known, as reported by Fox News, Johnson & Johnson is indeed less fast than Moderna and Pfizer which have obtained prior authorization for use. However, both vaccines are made with a two-dose administration model. while the Johnson & Johnson vaccine is only one dose.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)