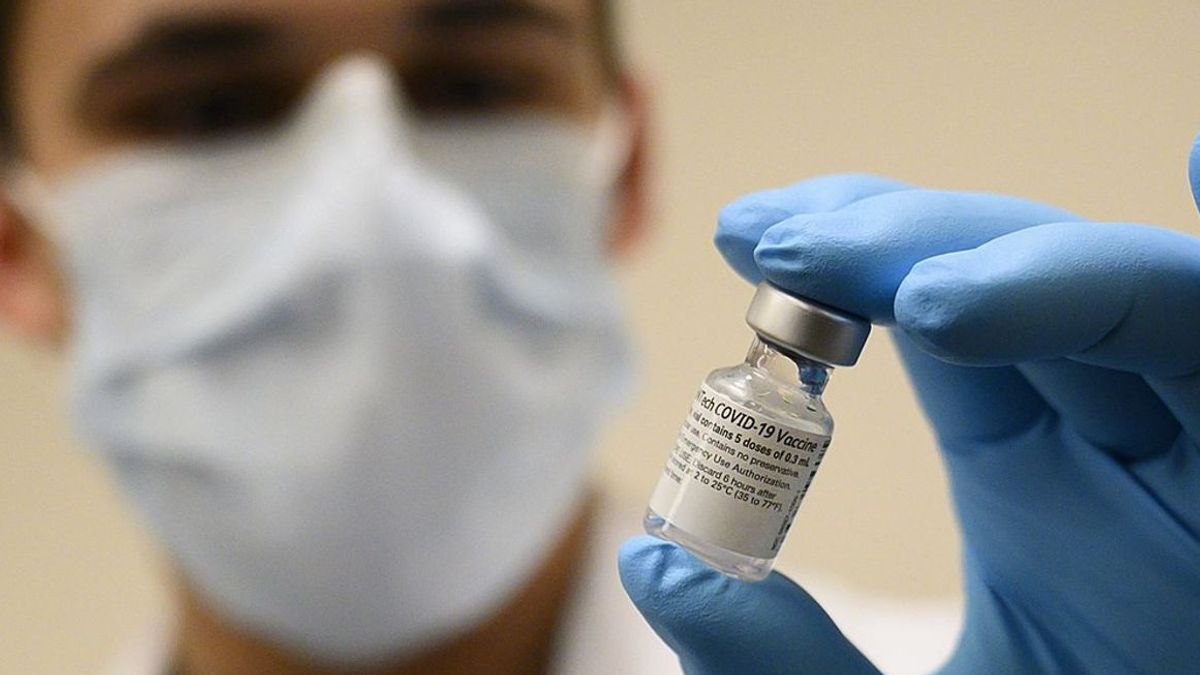
JAKARTA - The Food and Drug Administration (BPOM) has conducted sampling and testing of the Covid-19 vaccine made by Sinovac. This includes issuing Lot Release certificates for 1.2 million vaccines in accordance with an Emergency Use Authorization (EUA).
"During the admission process at the airport, the POM checks the suitability of documents, as well as the suitability of the temperature where the coronavac vaccine is stored," said Lucia Rizka Andalusia, spokesperson for Covid-19 Vaccination, in her official statement, Tuesday, January 5.
In addition, BPOM will also soon issue a lot release certificate for 1.8 million doses of the Covid-19 vaccine from Sinovac, which will arrive on December 31, 2020. A Lot Release Certificate is an important requirement that must be met in ensuring vaccine quality.
This requirement is a standard set by the World Health Organization (WHO), namely in the form of an evaluation process carried out by drug authorities in each country to ensure the quality of each lot or each batch of the vaccine.
"For the issuance of this certificate, the POM will conduct tests at the laboratory of the National Food and Drug Testing Development Center," said Rizka.
Lucia explained that this step was taken to accelerate the issuance of the EUA Covid-19 vaccine. In which BPOM will carry out rolling submissions so that data held by the pharmaceutical industry can be submitted gradually.
The BPOM has also evaluated preclinical test data, phase 1 and phase 2 clinical trials to assess the safety and immune response of vaccine use. The BPOM will also monitor the results of the phase 3 clinical trial within a period of 1 month after the second injection.
"Of course, according to the requirements of WHO, the minimum observation must be carried out up to 3 months for interim analysis. That will be used to obtain data on the safety and efficacy of vaccines as supporting data for EUA administration," he concluded.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)