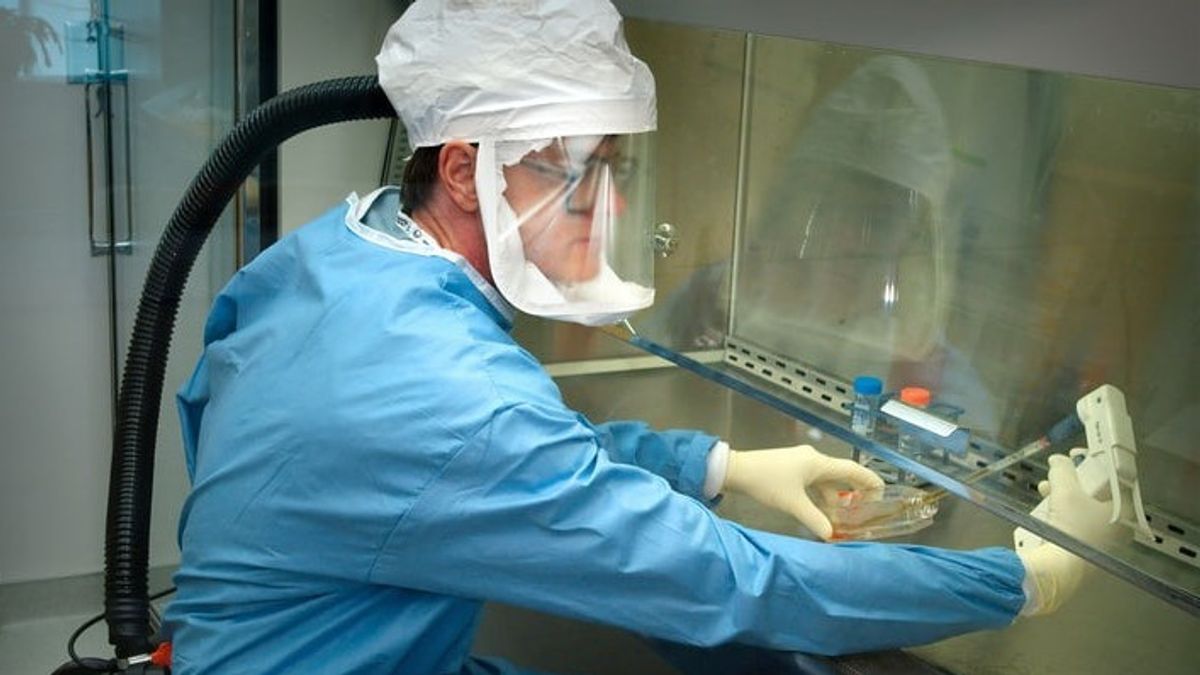
JAKARTA - Nearly one million people have been given the experimental COVID-19 vaccine developed by the Chinese pharmaceutical giant, Sinopharm. This is part of the emergency use program endorsed by the Chinese Government.
"In emergency use, we have now used it on nearly a million people. We have not received any reports of serious adverse reactions, and only a few have had mild symptoms," said Sinopharm chairman Liu Jingzhen.
Citing CNN, Saturday, November 21, Liu said the vaccine had been given to Chinese construction workers, diplomats and students who traveled to more than 150 countries around the world during the COVID-19 pandemic.
Most of them did not report having contracted COVID-19. Liu also said that on November 6 there were 56,000 people who had received emergency vaccinations and then went abroad.
For example, a transnational company has 99 employees at one of its offices abroad, 81 of whom are vaccinated. Then, an epidemic broke out in the office, 10 out of 18 people who were not vaccinated were infected and who were vaccinated did not catch COVID-19.
Sinopharm has so far conducted Phase three clinical trials involving nearly 60,000 people in 10 countries, including the United Arab Emirates, Bahrain, Egypt, Jordan, Peru and Argentina. Sinopharm has two vaccine candidates, but it is not clear which one has been widely administered.
China has given approval to companies to carry out vaccinations because of the urgency. Vaccination using an experimental vaccine that has only been tested for several months.
In June, Chinese company CanSino Biologics announced it had been given special authorization to deliver its experimental vaccine to the People's Liberation Army.
Since July, Chinese drugmakers have administered experimental vaccines to people working in "high-risk" professions under emergency use programs. The program allows trial vaccines to be used on a limited scale before their safety and efficacy are fully proven by clinical trials.
An experimental COVID-19 vaccine has even been reported to have been offered to the public in parts of China, causing several citizens to rush across the country to get the vaccine.
Another vaccine, Pfizer, has also announced the results of a Phase 3 COVID-19 vaccine trial at which it is said to be 95 percent effective in preventing transmission of COVID-19, even in the elderly and not causing serious problems. Another pharmaceutical company, Moderna, also announced that its COVID-19 vaccine is 94.5 percent effective.
Pfizer is expected to seek permission for the US Food and Drug Administration's emergency use for its vaccine. Anthony Fauci, a leading expert on infectious diseases in the US, said the Pfizer and Moderna vaccines had shown remarkable efficacy.
"That's amazing," Fauci said. "(The vaccine) is pretty much what we saw with measles (vaccine), which is 98 percent effective."
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)