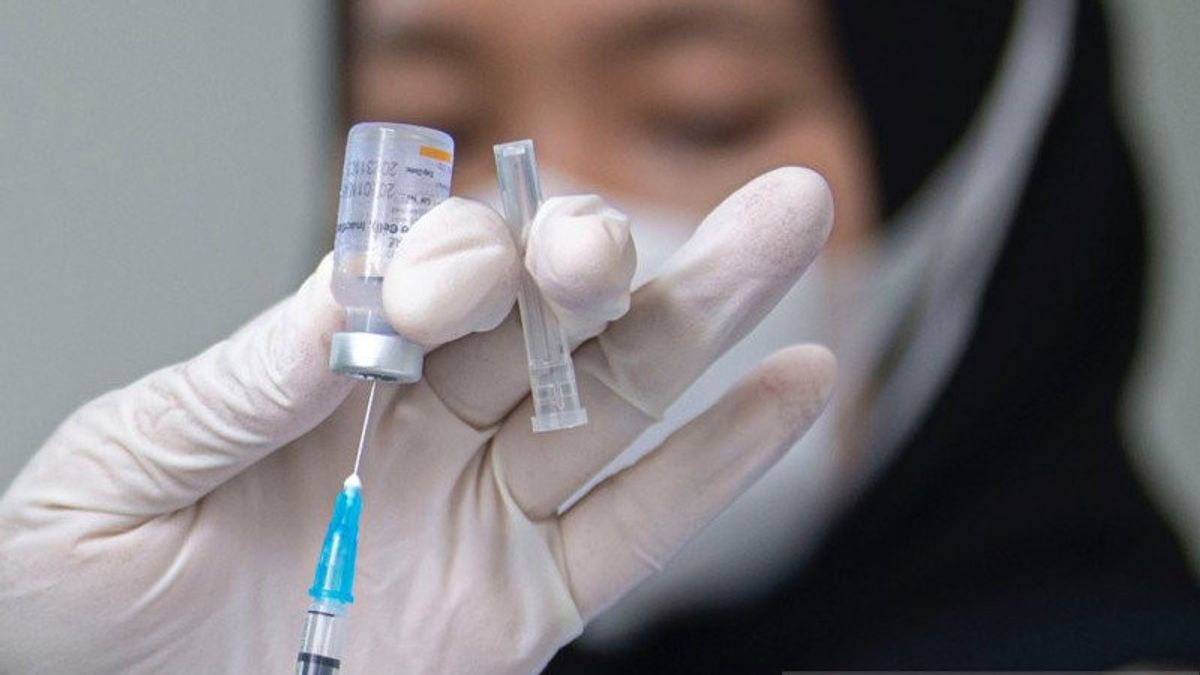
JAKARTA - The clinical trial of the Red and White vaccine at Airlangga University with PT Biotis Pharmaceuticals Indonesia began today. The clinical trial was conducted at dr. Sutomo Surabaya.
Member of Commission IX of the DPR, Yahya Zaini, hopes that the clinical trial of the Red and White vaccine can run smoothly and successfully. Because according to him, testing this vaccine is a history for the progress of the world of health in the country.
"If the first and second phases go well, it will be continued with the third phase in April 2022 with an even bigger subject. We all hope that the clinical trial will run smoothly and successfully, so that we will have independence in the field of vaccines," said Yahya Zaini, Wednesday, February 9.
According to the Golkar politician, Indonesia could have its own production vaccine with the completion of the Red and White Vaccine clinical trial. In this way, Indonesia has independence in the field of vaccines and its dependence on imported vaccines can be reduced.
"Considering that in 2021, we spent a budget of around Rp. 74 trillion for imported vaccines. If we could produce our own how much foreign exchange we could save on vaccine spending," he said.
The legislator from the VIII East Java electoral district said he was proud because the Red and White Vaccine made by Universitas Airlangga (Unair) was the first in Indonesia and even in Southeast Asia. It is estimated that Airlangga's vaccine production is not only to meet domestic needs, but also for export.
In addition, said Yahya, the vaccine made by Unair can also be used for primary vaccines and booster vaccines or injections of the third dose.
“As an alumni of Unair, I give my appreciation to the Unair Chancellor, academics and researchers who have worked tirelessly, even with a limited budget. The extraordinary scientific dedication has become a strong motivation to produce innovative works for the nation," he concluded.
It is known that the first phase of the Red and White Vaccine clinical trial involved 90 subjects, while the second phase consisted of 405 subjects.
Previously, BPOM had given approval for the Clinical Trial Protocol (PPUK) for the Red and White Vaccine developed by Unair based on the inactivated virus.
BPOM is expected to issue an Emergency Use Authorization (EUA) in July 2022 if clinical trials run smoothly. So far, BPOM has also provided guidance in the form of meeting standards and requirements to produce safe, efficacious, and quality drugs and vaccines produced by pharmaceutical industry facilities that meet international standard Good Manufacturing Practices (GMP) requirements.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)