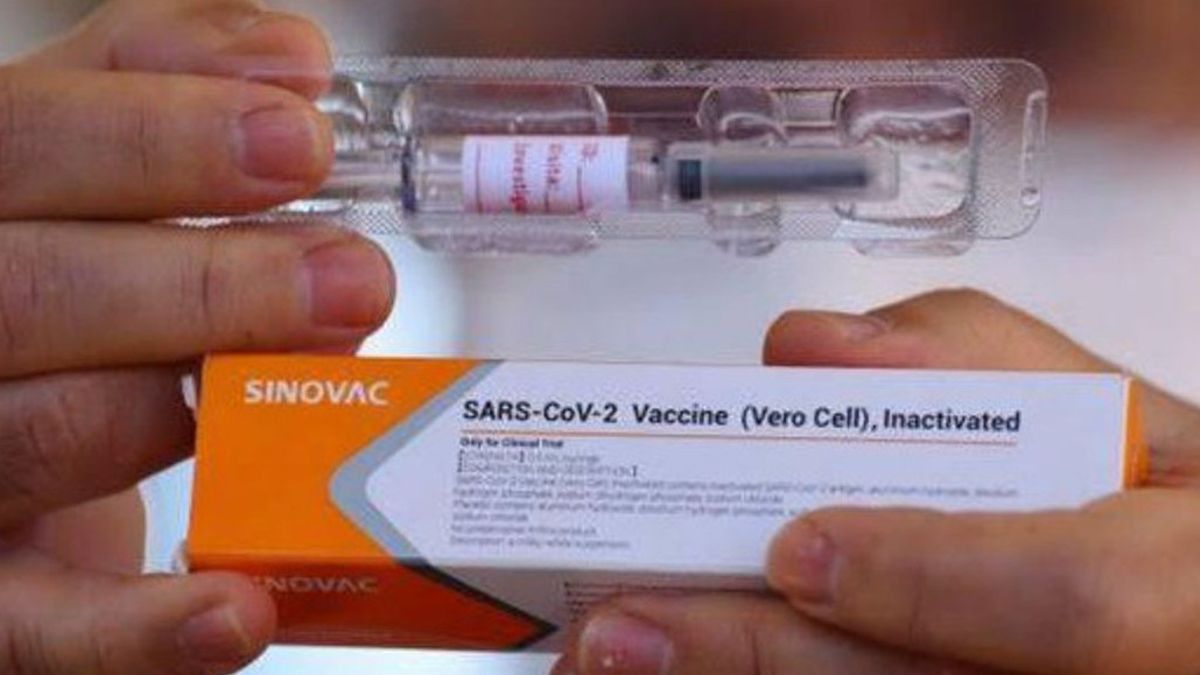
JAKARTA - The Twitter scene was enlivened by talks about the COVID-19 vaccine made by Sinovac. The @EnggalPMT account shared a picture showing Sinovac's white orange stripe with the words "Only for Clinical Trial".
In that tweet @EnggalPMT questioned how could the government force vaccination of its citizens to use a vaccine that should only be for clinical trials.
@EnggalPMT's tweet was viral, with Retweet activity 1,053 times. Not a few netizens also question why the government is giving this clinical trial vaccine to the public.
"Could it be that because I already bought myself afraid of losing? I ASK LOH ... GA NUDUH MACEM2," wrote @HasmiSahar.
"Wow ... So TRIAL RATS ... Hmmm ...," @ sonny140774.
"Alhamdulillah, there are also those who want to discuss this. I shook my head reading the information in the box yesterday," @ WongKit78352356.
"This is actually the case. Honestly, as the public, there is not enough information, especially regarding whether there will be side effects. Because the one who is smeared is really good at preventing and curing those who get COVID-19. I'm especially afraid of not being a guinea pig, honestly scared & terrified, "@ riewayne29 expressed concern about what she saw.
The Ministry of Health (Kemenkes) has clarified this. Contacted via text message, Ministry of Health's COVID-19 Vaccination Spokesperson, Siti Nadia Tarmizi, emphasized that images that are viral on social media are false. He said the COVID-19 vaccine that would be circulated and injected by the public was not shaped like that.
"What I know is that the vaccine that will be used is not the packaging, yes. (The packaging) is already in the form of a vial, yes," he told VOI, Saturday, January 2.
Nadia also clarified the words "clinical trial" in a viral picture. "And there's no 'clinical trial' ... That's for yesterday's Phase 3 clinical trial."
About the Sinovac vaccineSinovac vaccine is a vaccine produced by a pharmaceutical company from China. The Food and Drug Supervisory Agency (BPOM) is conducting further supervision and research on this vaccine at PT Bio Farma, Bandung.
That said, the resulting data showed good signs in terms of efficacy. Apart from Indonesia, the Sinovac vaccine is also being tested in clinical trials in Brazil, Turkey and Chile.
"Until now, the data has shown good results so this continues to increase our confidence as evaluators so that the results will be good," said Head of BPOM Penny K Lukito, written by Tempo.co, Thursday, December 31, 2020.
Here are four information about the Sinovac vaccine that we collect from various sources.
1. Approval out 1-2 weeksIn his latest statement regarding the Sinovac vaccine, Health Minister Budi Gunadi Sadikin said that approval could be issued within one to two weeks. "I feel that the first stage of vaccine supply and approval (Sinovac) can be completed in 1-2 weeks," said Budi.
Upon approval, the authorities, including the Ministry of Health, can begin distribution throughout Indonesia. The distribution will be carried out in a fast and short time, said Budi. He continued that the vaccination process will be prioritized for health workers and public workers.
"The most complex thing will be the final stage of how we can inject this vaccine at all service points for all people," he said.
Budi said, the final stage of the process could not only rely on the Ministry of Health. All stakeholders are obliged to work together so that the national vaccination program can become a mass movement throughout Indonesia.
2. Indonesia brought a total of 3 million dosesIndonesia has imported around three million doses of vaccine. In the first batch, the vaccine was delivered in a total of 1.2 million doses. The next flight group arrived at the end of last year, Thursday, December 31, 2020.
The dose in the second batch came in the amount of 1.8 million. All vaccines are currently being tested by BPOM. "With this arrival, 3 million Sinovac vaccines have been in Indonesia," said Foreign Minister Retno Marsudi.
3. Clinical trials in other countriesIn the process of the Sinovac vaccine clinical trial, Indonesia shared data with Brazil and Turkey. The two countries have provided clinical trial results that show good results.
Head of BPOM Penny K Lukito explained that from the results of the communication, clinical trial data in Brazil and Turkey showed consistently good results. The good results, he said, matched the tests carried out at the PT Bio Farma laboratory in Bandung.
"Until now, the data shows good results so this continues to increase our confidence as evaluators so that the results will be good," said Penny.
4. No side effectsPenny further claimed that there were no reports of side effects. Data shown by Brazil, Turkey, or from Bandung shows the effectiveness of vaccines against COVID-19.
"The results of temporary clinical trial data from observations after the completion of the second injection and observations for 1, 3 and 6 months have been given gradually," said Penny.
BPOM, said Penny, has also conducted an evaluation so that the Emergency Use of Authorization permit for the vaccine can be issued quickly.
"It has shown good data related to safety aspects and it has been reported that there are no serious side effects, thus showing a safety aspect that is consistent with the results during phase 1 and 2," he said.
The English, Chinese, Japanese, Arabic, and French versions are automatically generated by the AI. So there may still be inaccuracies in translating, please always see Indonesian as our main language. (system supported by DigitalSiber.id)